Lene A. H. Haakstad, MSc, PhD and Kari Bø, PhD
From the Department of Sports Medicine, Norwegian School of Sports Sciences, Oslo, Norway
OBJECTIVE: To determine whether participation in a group fitness class for pregnant women can prevent and treat pelvic girdle pain and low back pain.
DESIGN: An observer-blinded randomized controlled trial.
PARTICIPANTS: A total of 105 sedentary, nulliparous pregnant women, mean age 30.7 years (standard deviation (SD) 4.0), mean pre-pregnancy body mass index 23.8 (SD 4.3), were assigned to either control or exercise groups at mean gestation week 17.7 (SD 4.2).
METHODS: The exercise intervention followed the guidelines of American College of Obstetricians and Gynecologists and included a 60 min general fitness class, with 40 min of endurance training and 20 min of strength training including stretching, performed at least twice per week for a minimum of 12 weeks. Outcome measures were number of women reporting pelvic girdle pain and low back pain after the intervention (mean pregnancy week 36.6 (SD 0.9)) and postpartum (mean 7.7 (SD 1.7)).
RESULTS: There were no statistically significant differences between the exercisers and controls in numbers reporting the 2 conditions after the intervention (pelvic girdle pain: odds ratio (OR) = 1.34, CI = 0.56–3.20 or low back pain: OR = 1.10, CI = 0.47–2.60) or postpartum (pelvic girdle pain: OR = 0.38, CI = 0.13–1.10 or low back pain: OR = 1.45, CI = 0.54–3.94). A comparison of the women who had attended at least 80% of the weekly exercise classes with the control participants did not change the results.
CONCLUSION: Participation in regular group fitness classes during pregnancy did not alter the proportion of women reporting pelvic girdle pain or low back pain during pregnancy or after childbirth.
Key words: exercise; low back pain (LBP); pelvic girdle pain (PGP); pregnancy.
J Rehabil Med 2015; 47: 00–00
Correspondence address: Lene A. H. Haakstad, Norwegian School of Sport Sciences, Department of Sports Medicine, PO Box 4014, Ullevål Stadion, NO-0806 Oslo, Norway. E-mail: lahaakstad@nih.no
Accepted Aug 14, 2014; Epub ahead of print Nov 6, 2014
INTRODUCTION
Being pregnant is followed by increased body mass, as well as several changes in the musculoskeletal system, with possible subsequent pregnancy complaints (1). To date, pregnant women constitute one-third of all sick-leave for women aged between 20 and 39 years, and by 32 weeks of gestation, 63% of Norwegian women are on sick-leave (2). According to Dørheim (2), pelvic girdle pain (PGP) accounts for most of the sick leave in pregnancy in Northern European countries. In Norway, the mean sick-leave due to PGP during pregnancy was 12 weeks and reported prevalence was 32% (2). Compared with the non-pregnant state, there is also a significant increase in women reporting low back pain (LBP) during pregnancy (3). According to Pennic & Liddle (4), approximately two-thirds of pregnant women report LBP. For both conditions, typically, the pain worsens as pregnancy advances, and negatively influences work, normal sleep, as well as activities of daily living and physical activity level (4, 5). For some women, the pain is still persistent several years postpartum (6, 7). Consequently, both PGP and LBP have large socioeconomic implications (8) as well as significant impact on physical and psychological quality of life for the women and their families (9).
Recommendations for exercise during pregnancy suggest that, in the absence of medical and obstetric complications, pregnant women should aim to perform at least 30 min or more of moderate intensity physical activity daily, and/or exercise 3–5 times weekly for a minimum of 15–30 min (10, 11). To date, there is scant knowledge and inconsistent results regarding the influence of regular exercise on PGP and LBP during pregnancy (4). A Cochrane review from 2013, showed that physiotherapy, acupuncture, use of pelvic belts and pillows, as well as aquatic and stabilization exercise programmes seemed to relieve pelvic or back pain more than usual prenatal care (4), with no clear consensus regarding the best method for prevention or treatment of the 2 conditions (12–14). Methodological concerns included small sample sizes, lack of randomization, no blinding of assessors, incomplete outcome data, as well as insufficient information on baseline equality between groups (4).
The primary aim of the present study was to examine whether participation in a group fitness class for pregnant women twice a week, in addition to 30 min of moderate self-imposed physical activity on the remaining week-days, can prevent and treat PGP and LBP in previously inactive women.
METHODS
Design
The study design was a secondary analysis of an assessor-blinded RCT, with the primary aim of evaluating the effect of regular exercise on maternal weight gain (15). The complete study was conducted in agreement with the most recent CONSORT statement (http://www.consort-statement.org) and was registered in the ClinicalTrials.gov Protocol Registration System (NCT00617149).
Participants
Nulliparous women whose pre-pregnancy exercise levels did not include participation in a structured exercise programme (> 60 min once per week), including brisk walking (> 120 min per week) for the past 6 months, were eligible for the trial. Other inclusion criteria were ability to read, understand and speak Norwegian language, and to be within their first 24 weeks of pregnancy. Exclusion criteria were a history of more than 2 miscarriages, severe heart disease (including symptoms of angina, myocardial infarction or arrhythmias), persistent bleeding after 12 weeks of gestation, multiple pregnancy, poorly controlled thyroid disease, pregnancy-induced hypertension or pre-eclampsia, diabetes or gestational diabetes, and other diseases that could interfere with participation (11). In addition, women not able to attend weekly exercise classes were ineligible. Participants were recruited via articles and advertisement in newspapers, by health practitioners (family physicians, midwives) and websites for pregnant women.
In total, 105 women were recruited to the trial from February to May 2008. The majority of participants came from the city of Oslo, Norway. All follow-up procedures were completed by March 2009. An a priori power calculation was made for the primary outcome of the trial, which was gestational weight gain (15).
In total, the participants were examined with respect to the current outcome measures 3 times during the study period. The first visit was between 12 and 24 weeks of gestation (baseline visit), the second at week 36–38 (after the intervention) and the last 6–8 weeks after delivery (postpartum visit). Each visit lasted approximately 60–75 min and included, in addition to standardized interviews for assessing health outcomes such as PGP and LBP, measurements of height and body weight, skinfold thickness and a submaximal lactate profile step test (treadmill walk test). There was no financial compensation to the participants.
All participants gave written consent to participate and the procedures followed the World Medical Association Declaration of Helsinki. The project was approved by The National Committee for Medical Research Ethics, Southern Norway, Oslo, Norway (reference number S-05208). The Norwegian Social Sciences Data Services (NNT) provided licence to store and register individual health information (reference number 17804/2/KH).
Randomization
A secretary, not involved in the assessment or exercise classes, assigned the participants to either an exercise group or a control group following a computerized statistical randomization program with sealed opaque envelopes. The procedure was simple randomization and no stratification was done. The principal investigator (LAHH) was not involved in training the women and was blinded to group allocation while assessing the outcome measures, plotting and analysing the data.
Intervention
Participants randomized to exercise were prescribed to participate in at least 2 out of 3 possible 1 h aerobic dance classes per week, for a minimum of 12 weeks. Each session started with 5 min warm up, followed by 35 min endurance training and aerobic dance, including cool down. This was followed by 15 min strength training with a special focus on the deep abdominal stabilization muscles (internal oblique and the transverse abdominal muscle), pelvic floor and back muscles. The last 5 min included stretching, relaxation and body awareness exercises. The aerobic dance routine included low impact exercises (no jumping or running) and step training. Step length and body rotations were reduced to a minimum, and crossings of legs and sharp and abrupt changes of position were avoided. The exercise programme followed the current American Congress of Obstetricians and Gynecologists (ACOG) exercise prescription (10, 16), and all aerobic activities were performed at moderate intensity measured by ratings of perceived exertion at 12–14 (somewhat hard) on the 6–20 Borg rating scale (17). The exercise programme was choreographed and led by certified aerobic instructors.
In addition to joining the scheduled aerobic classes, all women in the exercise group were asked to include 30 min of moderate self-imposed physical activity on the remaining week days. They were also advised to incorporate short bouts of activity into their daily schedules (e.g. to walk instead of drive short distances and to use stairs instead of lifts). Adherence to the exercise classes was reported by the aerobic instructors, and the self-imposed daily activity was registered in a personal training diary.
Control participants were neither encouraged to, nor discouraged from, exercising, as we considered asking the controls not to exercise to be against current guidelines. In order to treat the 2 groups identically apart from for the intervention, the control group underwent all tests and completed the same interview as the exercise group. Otherwise, the control group received usual prenatal care.
Outcome measures
Primary outcome measures were number of women reporting PGP and LBP at gestation week 36–38 and 6–8 weeks postpartum. Secondary outcomes included severity, defined as limitations in performing activities of daily life and physical activity.
Assessments of PGP and LBP were obtained as part of the questions concerning pregnancy complaints and included a yes or no response to one separate question for each condition, asked on 3 occasions: “Have you experienced PGP this week or in previous weeks? “Have you experienced back pain this week or in previous weeks?” If the participants answered yes to PGP and/or back pain, pain localization was investigated: “Where do you experience the pain?” The categorical responses for PGP were: in front (symphysis), back (1 side), back (2 sides), back and in front (1 side), back and in front (2 sides). For back pain the following 3 alternatives were provided: upper pain, LBP with pain radiating to the legs and LBP not radiating to the legs.
The interview questionnaire also contained 2 yes/no questions concerning the disability or severity of PGP/LBP (“Does the pain stop you performing daily activities at work and/or at home? Does the pain stop you performing regular physical activity/exercise?”). Furthermore, for the assessment of PGP, one additional question regarding the use of crutches was asked: “Do you have problems walking to the extent of using crutches?” The response options were: “Not at all, Seldom, Sometimes or Most of the day”. Severe PGP was defined as using crutches “Sometimes or Most of the day”.
The baseline interview also covered demographic information (e.g. age, pregnancy week, height, maternal weight gain weight, smoking habits, education, occupation, reports of being sick-listed) and assessment of physical activity and sedentary behaviour (at work, transportation and household). The questions on total physical activity have been validated with a portable activity monitor (18).
Data analysis
All statistical analyses were conducted with SPSS Statistical Software version 18.0 for Windows. Data are presented as numbers with percentages or means with standard deviation (SD). The principal analysis was done on an intention to treat basis (ITT). Missing values were replaced with values based on existing data (Last-Observation-Carried-Forward). In addition, we performed per protocol analysis based on adherence to ≥ 80% of the recommended exercise sessions (≥ 19 exercise sessions) and compared women with 100% exercise adherence (24 exercise sessions) with the control group. The differences in the proportion of women reporting PGP and LBP, as well as numbers reporting reduction in daily activities and physical activity level in the intervention and control group, were tested by two-sided χ2-test. For expected cell values less than 5, Fisher’s exact test was used. Binary logistic regression was used to estimate effect sizes and their 95% confidence intervals (95% CI). A p-value < 0.05 was considered statistically significant.
RESULTS
Background variables of the women randomized to either exercise or control groups are shown in Table I. There were no significant differences in background variables or prevalence rates of women with PGP or LBP in the exercise and control groups before the intervention (Table II). At baseline, prevalence rates of PGP and LBP for the whole group (n = 105) were 27.6% and 33.3%, respectively. For PGP, the majority of participants (63%) defined pain in the symphysis pubis only. LBP, upper back pain or a combination of both were reported by 77.8%, 17.8% and 4.4%, respectively. For both outcomes (PGP and LBP), no difference in pain localization was seen between exercise and control groups.
|
Table I. Background variables in the exercise and control groups (n = 105) |
||
|
Details |
Exercise (n = 52) |
Control (n = 53) |
|
Age, years, mean (SD) |
31.2 (3.7) |
30.3 (4.4) |
|
Gestational weeks, mean (SD) |
17.3 (4.1) |
18.0 (4.3) |
|
Married/living together, n (%) |
51 (98.1) |
52 (98.1) |
|
College/university education, n (%) |
44 (84.6) |
45 (84.9) |
|
Sedentary occupations (> 50% of the working day), n (%) |
37 (71.2) |
36 (67.9) |
|
Daily smokers, n (%) |
2 (3.8) |
1 (1.9) |
|
Height, m, mean (SD) |
1.69 (0.1) |
1.69 (0.1) |
|
Pre-preg weight, kg, mean (SD) |
67.9 (11.4) |
68.4 (14.6) |
|
Weight, kga, mean (SD) |
71.8 (11.4) |
72.7 (14.3) |
|
Pre-pregancy BMI, kg/m2, mean (SD) |
23.8 (3.8) |
23.9 (4.7) |
|
Pre-pregancy BMI ≥ 25, n (%) |
13 (25.0) |
14 (26.4) |
|
Sick-leave, days, mean (SD) |
10 (19.2) |
13 (24.5) |
|
aAt baseline test, pregnancy weight was measured using a digital beam scale. BMI: body mass index. |
||
|
Table II. Effect sizes with confidence intervals (CI) and numbers of women reporting pelvic girdle pain (PGP) and low back pain (LPB) before the intervention (gestation week 12–24), after the intervention (gestation week 36–38) and 6–8 weeks postpartum in the exercise and control groups |
||||||
|
PGP n (%) |
OR (95% CI) |
p-value |
LBP n (%) |
OR (95% CI) |
p-value |
|
|
Before the intervention |
||||||
|
Exercise (n = 52) Control (n = 53) |
14 (26.9) 15 (28.3) |
0.93 (0.40–2.20) |
0.87 |
15 (28.8) 17 (32.1) |
0.94 (0.42–2.13) |
0.89 |
|
After the intervention |
||||||
|
Exercise (n = 42) Control (n = 42) |
16 (38.1) 19 (45.2) |
1.34 (0.56–3.20) |
0.51 |
19 (45.2) 18 (42.8) |
1.10 (0.47–2.60) |
0.83 |
|
Postpartum |
||||||
|
Exercise (n = 43) Control (n = 47) |
6 (14.0) 14 (29.8) |
0.38 (0.13–1.10) |
0.07 |
8 (18.6) 5 (10.6) |
1.45 (0.54–3.94) |
0.47 |
|
OR: odds ratio. |
||||||
Nineteen percent in the training group and 20.8% in the control group were lost to follow-up. Fig. 1 shows the flow-chart and reasons for loss to follow-up reported in the 2 groups. Some women lost to the second visit, re-entered the study at the postpartum examination. Mean adherence to the exercise classes was 17.2 (SD 12.5, range 1–55) out of 24 recommended training sessions. Twenty-one women (40.4%) attended ≥ 80% of the training sessions.
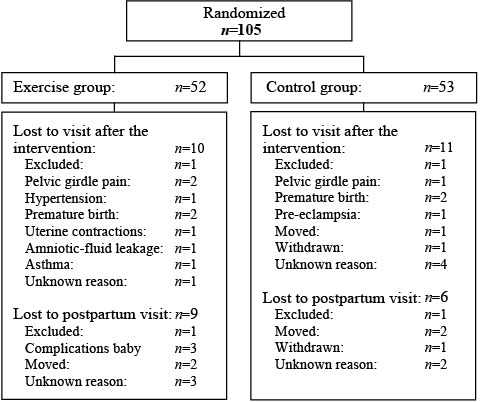
Fig. 1. Trial profile showing the flow of participants throughout the study period.
Sixty-two percent of participants returned their training diaries and reported daily minutes with physical activity and exercise. Excluding low intensity activity and the scheduled aerobic classes, the results showed a mean weekly exercise time of 90 min (SD 73) of moderate exercise, with 16 women (30.8%) following the current pregnancy exercise guidelines of a minimum of 15 min moderately intensity exercise, 3–5 times a week (19).
The prevalence of PGP and LBP before the intervention, (mean pregnancy week 17.7, SD 4.2), after the intervention (mean pregnancy week 36.6, SD 0.9) and postpartum (mean 7.7, SD 1.7) are shown in Table II. There was no statistically significant difference in the number of women reporting any of these conditions at any assessment point. In the exercise group, 14 (26.9%) and 15 (28.8%) women reported PGP and LBP at the onset of the intervention, respectively. This number increased to 16 (38.1%) women with PGP and 19 (45.2%) women with LBP after the intervention. Postpartum the prevalence was 6 (14.0%) with PGP and 8 (18.6%) with LBP, with a small tendency towards lower prevalence of PGP postpartum in the exercise group compared with the control group. As shown in Table III, a comparison of the women who had 100% exercise adherence or attended at least 80% of the weekly exercise classes with the non-participants did not change the results. Moreover, analysing the data according to whether the participants had PGP or LBP when commencing into the study did not change the results.
|
Table III. Effect sizes with confidence intervals (CI) and numbers of women (n (%)), reporting pelvic girdle pain (PGP) and low back pain (LBP) in the exercise and control group after the intervention period, analysed by intention to treat (ITT), per protocol (≥80% of exercise sessions) and analyses of 100% exercise adherence (24 exercise sessions) |
||||||||||||||
|
ITT |
Per protocol |
100% exercise adherence |
||||||||||||
|
EG (n = 52) n (%) |
CG (n = 53) n (%) |
OR (95% CI) |
p-value |
EG (n = 21) n (%) |
CG (n = 53) n (%) |
OR (95% CI) |
p-value |
EG (n = 14) n (%) |
CG (n = 53) n (%) |
OR (95% CI) |
p-value |
|||
|
PGP |
18 (34.6) |
21 (39.6) |
0.81 (0.37–1.78) |
0.60 |
9 (42.9) |
21 (39.6) |
1.21 (0.757–1.92) |
0.43 |
6 (42.9) |
21 (39.6) |
1.159 (0.75–1.79) |
0.51 |
||
|
PGP have a negative effect on activities of daily life |
9 (50.0) |
11 (52.4) |
0.92 |
4 (44.4) |
11(52.4) |
0.51 |
2 (33.3) |
11 (52.4) |
0.30 |
|||||
|
PGP have a negative effect on PA |
13 (72.2) |
14 (66.7) |
0.59 |
6 (66.7) |
15 (71.4) |
0.70 |
5 (83.3) |
14 (66.7) |
0.63 |
|||||
|
LBP |
19 (36.5) |
18 (34.0) |
2.60 (0.27–25.99) |
0.95 |
9 (42.9) |
18 (34.0) |
1.00 (0.16–6.26) |
0.90 |
7 (50.0) |
18 (34.0) |
1.74 (0.33–9.19) |
0.51 |
||
|
LBP have a negative effect on activities of daily life |
3 |
1 |
0.40 |
2 |
1 |
0.09 |
1 |
1 |
0.11 |
|||||
|
LBP have a negative effect on PA |
1 |
1 |
0.88 |
0 |
1 |
0.82 |
0 |
1 |
0.94 |
|||||
|
*Some of these numbers are too small for meaningful statistics. OR: odds ratio; EG: experimental group; CG: control group; PA: physical activity. |
||||||||||||||
No significant differences were found in the secondary outcomes regarding disability and severity of the complaints, and no women with PGP reported serious limitations in daily life activities, defined as using crutches “Sometimes or Most of the day”. Furthermore, there was no significant difference between the exercise and control groups in reported sick-leave related to PGP (15.3% vs 17.0%, p = 0.6) and LBP (13.5% vs 13.2%, p = 1.0) after the intervention.
DISCUSSION
To our knowledge, this is one of few RCTs investigating the effect of implementing supervised group exercise with emphasis on cardiovascular endurance training and muscular strengthening exercise in previously sedentary women on PGP and LBP. We found no statistically significant difference between the intervention and control groups in prevalence of the 2 conditions at any assessment points. On the other hand, no negative effects of the 12-week intervention were reported.
The strengths of the present study included the use of an assessor-blinded RCT design and implementation of an exercise programme following ACOG recommendations (10). The same primary investigator examined all the participants using a standardized questionnaire including subgrouping of PGP and LBP. In addition, the presence of qualified instructors for guidance and supervision, as well as registration of exercise adherence both by the fitness instructors and via recordings in a training diary, may be considered strengths of the study. Study limitations include the sample size, which was not based on a priori power calculations for PGP and LBP outcomes, somewhat high loss to follow-up at post-test and low adherence to group exercises classes.
The prevalence rates of PGP and LBP for the whole group at first assessment were comparable to another Norwegian study evaluating the effect of supervised group exercise on PGP and LBP (20), as well as point prevalence of pregnant women with PGP and LBP, reported in the European guidelines for the diagnosis and treatment of pelvic girdle pain (1). However, the huge differences in interventions, time-point in pregnancy in the women recruited, measurement of outcomes and diagnosis of PGP, LBP and combined pelvic and back pain preclude comparisons of the results and estimates of study effect with other RCTs. Mørkved et al. (21) found a 12% and 11% difference in the prevalence of lumbopelvic pain in favour of the intervention group, at 36 weeks of gestation and 3 months postpartum, respectively. This study included less aerobic exercise and had pelvic floor muscle training as their main focus. Stafne et al. (22) showed no effect of regular exercise (aerobic activity, body strengthening and balance training) on prevalence of lumbopelvic pain, but a lower prevalence of women on sick-leave due to lumbopelvic pain. A limitation of both the above-mentioned studies is no differentiation between PGP and LBP. According to several studies, PGP is more common, especially from the second trimester, and has greater functional impairments than LBP (9, 23). In the present study PGP and LBP were separated with different questions, in accordance with the study of Bø & Backe-Hansen (24), showing that a standardized questionnaire performed in an interview setting are able to distinguish between these 2 conditions. Nevertheless, more detailed questions, including use of body charts and a possibility for the participant to give more information about the location, nature and extent of pain, may have made it easier to correctly classify the women into the 2 groups (PGP/LBP) and women with no PGP/LBP in the present study. To-date guidelines for classification of PGP are well known, yet, no universal agreement on how to differentiate PGP from LBP is established (1, 25).
The inclusion or exclusion of women with co-existing PGP/LBP and the definitions and classification systems used to measure PGP/LBP, have shown greatly to influence the prevalence estimates (26), as well as the reported effect size of the different interventions (20–22, 27). To our knowledge, only one previous RCT included measurement of both PGP and LBP, as well as included women with or without the 2 complaints at the baseline registration (20). This trial found no effect of supervised group exercise on PGP or LBP compared with the control group (20). Our results support this conclusion. A recent Cochrane review, involving 4,093 women in 26 trials, investigating the effects of exercise (land- or water-based), pelvic belts, acupuncture, manual therapy and education, concluded that the available data were insufficient to infer important benefits of any treatment given (4).
Loss to follow-up at post-test and low adherence to exercise may have reduced the power of the present study and the ability to draw clear conclusions. Imputation techniques can never compensate for, or exactly reproduce, missing data (28). We used the last observation carried forward (LOCF) method to achieve a complete data-set and minimize the number of participants to be eliminated from the analysis. The basic assumption underlying LOCF is that participants who are given treatments (e.g. participate in regular exercise) improve, which makes treating missing data as if the past had continued unchanged, rather conservative. The strength of LOCF and ITT analysis is that it promotes balance between intervention groups for both known and unknown confounders, and thereby preserves the benefits of randomization (28).
Adherence is defined as to what degree study populations act in accordance with advice given by their researcher or medical doctor (29), and poor adherence is considered to limit the effect of different interventions (i.e. exercise does not give results if you do not do it). Currently, all healthy pregnant women are advised to participate in regular exercise throughout pregnancy (10). In the present study, we emphasized the importance of adherence to the exercise protocol. In addition, each participant in the exercise group was asked to complete a home training diary to record all physical activity on the non-supervised weekdays. The use of exercise diaries has previously shown to increase adherence to home exercise, as keeping records may help individuals to become more aware of what they are doing, how much, and whether they are meeting weekly exercise goals (29). Despite this, only 40% attended the recommended exercise classes at the university, and the somewhat low rate of handing in the training diaries, reduced the intention to analyse data on adherence to physical activity at home with respect to study end-points. Moreover, data was also missing in the diary records, especially after long-term use.
A fitness class of 60 min prescribed at least twice a week, including endurance training of 35–40 min may be considered demanding. Thus, the sedentary women being the target group for this study may have been less motivated to adhere to this specific programme. In addition, finding time to exercise is vital if an exercise programme is to be adhered to. Even though the exercise groups were arranged in the evenings, previously sedentary women may have had problems adhering to a weekly exercise routine. Analysis of the data for women attending at least 80% of the recommended exercise classes did not change the results, but this analysis is unfortunately limited by small numbers. A power calculation based on the present results, with 80% power and p < 0.05, showed that a prevalence reduction from 40% to 20%, would require approximately 90 participants in each group to show statistically significant differences. More studies investigating the effect of a general group fitness class for pregnant women with a similar population-based approach are needed. The results of the present study may serve as a basis for power calculations in future research.
A group training setting might not be considered ideal for PGP/LBP prevention or treatment. To date, the recommended treatment for pregnancy-related PGP and LBP, includes adequate information and reassurance of the patient, as well as individualized exercises (1, 4). Even though the participants of the exercise group were limited to a maximum of 20, it can be questioned whether the training was sufficiently tailored to each individual. Moreover, the exercise programme was not specifically designed for treating or preventing PGP or LBP in pregnant women, and involved weight-bearing exercises, which may increase the forces across the pelvic joints and the lower extremities, especially in combination with increased maternal weight gain. Accordingly, it has been speculated that engaging in weight-bearing activities might lead to PGP or lumbopelvic pain (30). In the present study, PGP/LBP was not more frequently reported in the exercise group compared with the control group, and PGP as a cause for drop-out was described by 2 and 1 participants in the exercise and control groups, respectively. From a health promotion and prevention point of view, it would have been advantageous if a general group fitness class for pregnant women had been successful, as this would have been less time-consuming, more cost-effective and possibly more motivating than one-to-one exercise with a healthcare professional.
The present RCT had a pragmatic preventive approach and included participants with and without PGP and LBP by inclusion. This is in agreement with Eggen et al. (20). It was therefore considered important to recruit a heterogeneous population, reflecting the variation between pregnant women that occur in real-life settings to whom the treatment will be applied. Study participants were contacted across a wide range of sites and settings, varying from newspapers, flyers, maternity clinics and word of mouth. This is in contrast to most other studies, where the pregnant women have been recruited from 1 or 2 maternity units only (20–22). However, RCTs are time-consuming and involve cooperation from the participants. Therefore, pregnant women who volunteer for such a study may have an interest in, and be more attentive to, exercise than non-participants, thus creating a potential risk for selection bias. The pregnant women in this study were healthy nulliparous with a high educational level, and are therefore not representative for all eligible women.
In conclusion, a group fitness class for pregnant women twice a week with focus on cardiovascular endurance training and strength training had no effect on the proportion of previously inactive women reporting PGP and LBP during pregnancy or at 6–8 weeks postpartum. Further studies on strategies to achieve adherence to exercise protocols among previously sedentary pregnant women are warranted.
ACKNOWLEDGEMENTS
Thanks to Professor Ingar Holme for assistance with the statistical analysis. The present study was financed by, and conducted at the Norwegian School of Sport Sciences, Department of Sport Medicine, Oslo, Norway. There are no competing interests or financial disclosure.
REFERENCES
