Kumiko Ito, MSc1, Masahiro Kohzuki, MD, PhD1, Tamao Takahashi, MD1 and Satoru Ebihara, MD, PhD1,2
From the 1Department of Internal Medicine and Rehabilitation Science, Tohoku University Graduate School of Medicine, Sendai and 2Department of Rehabilitation Medicine, Toho University Graduate School of Medicine, Tokyo, Japan
Objective: Weight loss is common in patients with chronic obstructive pulmonary disease (COPD). Anorexia, postulated to be associated with alteration in taste sensitivity, may contribute to weight loss in these patients. Pulmonary rehabilitation is known to lead to improved exercise performance in patients with COPD. However, the relationship between pulmonary rehabilitation and taste sensitivity has not been evaluated. The objective of this study was to compare taste sensitivity before and after pulmonary rehabilitation in patients with COPD.
DESIGN: Single-group intervention trial.
Patients: Twenty-two patients with COPD.
METHODS: The six-min walk distance (6MWD), COPD assessment test, body mass index, fat mass index, fat-free mass index and taste test were conducted before and after 4-week pulmonary rehabilitation. Taste sensitivity was evaluated using the filter-paper disc method for 4 taste stimuli. Taste stimuli were salty, sweet, sour, and bitter tastes. Taste sensitivity was evaluated before and after pulmonary rehabilitation using the taste recognition threshold.
RESULTS: Following pulmonary rehabilitation, the 6MWD, COPD assessment test, salty recognition threshold, sweet recognition threshold and bitter recognition threshold improved significantly, whereas there were no significant improvements in body mass index, fat mass index, fat-free mass index or sour recognition threshold.
CONCLUSION: Pulmonary rehabilitation may improve taste sensitivity in patients with COPD.
Key words: chronic obstructive pulmonary disease; pulmonary rehabilitation; taste.
J Rehabil Med 2014; 46: 00–00
Correspondence address: Satoru Ebihara, Department of Rehabilitation Medicine, Toho University Graduate School of Medicine, 6-11-1 Omori-nishi, Ota-ku, Tokyo 143-8541, Japan. E-mail: satoru.ebihara@med.toho-u.ac.jp
Accepted Apr 25, 2014; Epub ahead of print Aug 28, 2014
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) causes disability and death worldwide (1). COPD is a disease characterized by airflow limitation, that is not fully reversible (1). The airflow limitation is usually progressive and is associated with an abnormal inflammatory response of the lungs, caused by noxious particles, gases and cigarette smoking. Although COPD affects the lungs, it also has significant systemic consequences (2).
The chronic course of COPD exhibits not only pulmonary structural and functional impairment, but also metabolic, hormonal and systemic organ dysfunction, such as in the skeletal muscle, heart, brain and skeleton (3). Inflammatory activation in COPD induces a hypermetabolic state, characterized by catabolic and anabolic imbalance, which results in weight loss (4), commonly seen in patients with COPD. Weight loss and low body weight are independent risk factors of morbidity and mortality in such patients (5). Recurrent exacerbation is associated with worsening of lung function and decreased survival (6).
Weight loss occurs when energy intake is inadequate to meet energy expenditure. One possible reason for weight loss in patients with COPD is altered taste sensitivity. Because patients with COPD may need to consume additional energy to maintain or gain weight, the taste sensory quality of meals becomes important. Studies have shown alteration in taste sensitivity to be an effect of several diseases, and some of these studies have found a relationship between alteration in taste sensitivity and anorexia, decreased food consumption and weight loss (7).
Anorexia may contribute to weight loss in patients with COPD (8). These patients may not consume adequate energy for a variety of reasons. The cause of poor food intake may be related to alteration in taste thresholds, a relationship demonstrated in both normal individuals and in patients with a variety of malignant tumours (9). Underweight patients with COPD (body mass index (BMI) < 21 kg/m2) have been reported to have a significantly higher bitter taste threshold than normal-weight patients (10), and alteration in taste sensitivity has been shown to be related to anorexia, decreased food consumption and weight loss (11). In patients with COPD, medications may affect taste. Taste disturbance affects nutritional status. Bronchodilators, such as ipratropium, fluticasone and salmetrol, have possible side-effects, including dry mouth and bitter taste (12). Dry mouth alters the taste sensitivity and taste threshold (13).
It has been shown that, in patients with COPD, maximal incremental cycle ergometer exercise significantly affects muscle aerobic capacity and exercise tolerance (14). It has also been reported that patients with COPD who participated in a standardized inpatient exercise training programme, consisting of daily submaximal cycle ergometry, treadmill walking, weight training and gymnastics, for a period of 8 weeks, experienced significant weight increase (15). However, the relationship between alteration in taste recognition threshold in patients with COPD and exercise has not yet been evaluated. Knowledge about whether alteration in taste sensitivity occurs in patients with COPD would have clinical implications for those dealing with such patients.
The objective of this study was to determine whether there were taste recognition threshold differences between the beginning and end of a pulmonary rehabilitation (PR) programme in patients with COPD. Since malnutrition is known to affect taste sensitivity in general (11), we recruited patients without emaciation in order to investigate the direct effect of exercise training on taste sensitivity in COPD.
METHODS
Subjects
Twenty-two patients (17 males, 5 females, mean age 72.4 years old (standard deviation (SD) 8.6)) hospitalized at Tohoku University Hospital from April 2011 to December 2012 with a diagnosis of COPD were enrolled in the study. All subjects were diagnosed by pulmonary specialists on the basis of a spirometry test, as well as according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines (16). The study was conducted according to the principals of the Declaration of Helsinki and was approved by the Tohoku University Ethics Committee Sendai, Miyagi, Japan (2011-185). Subjects gave written informed consent prior to the study.
Intervention
The intervention was a pulmonary rehabilitation (PR) programme; an evidence-based support programme providing comprehensive intervention for chronic respiratory disease to improve the condition of patients and increase their exercise tolerance (17). The programme includes breathing strategies, exercise training, nutritional counselling, and psychological counselling administered by doctors, nurses, physical therapists, occupational therapists, and dieticians (18). Nutritional counselling involves educating patients about estimated energy requirements based on basal energy expenditure taking into consideration physical activity energy expenditure (19). Each patient with COPD took part in the PR programme, which consisted of a 20-min class one or more times a day. The classes were conducted 5 days a week by physiotherapists for 4 weeks. The staff of the PR programme taught the subjects breathing techniques and exercise according to pulmonary function and exercise tolerance of each subject at the beginning of the PR programme. The exercise training consisted of walking on a treadmill, stair climbing, and ergometer cycling. Frequency of the training programme was 5 times a week for 4 weeks. The intensity of the training programme was 60–70% of peak work rate. The duration of the training programme was 20 min (20).
Procedures and evaluation
Evaluation of the PR programme was based on BMI, pulmonary function, 6MWD, COPD assessment test (CAT), and the filter-paper disc method. BMI was calculated using the patient’s height and weight (in kg/m2) defined as weight (in kg) divided by the square of height (in m). Body weight was assessed to the nearest 0.1 kg, with subjects standing in their bare feet and in light clothing. Height was measured by a clinical stadimeter with the patient in bare feet. Body composition was assessed using bioelectrical impedance analysis (BIA; In Body S20, Kobe Medicare, Kobe, Japan). BIA was conducted by dieticians. Fat mass index (FMI) was calculated as weight/height2 (kg/m2) (21). Fat free mass index (FFMI) was calculated as weight/height2 (kg/m2) (21).
Pulmonary functions were evaluated using spirometry for forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1) and FEV1/FVC (%). Physiotherapists conducted the 6MWD in patients with COPD (22). The 6MWD, which is a widely used, reliable submaximal exercise test that assesses the physiological and functional status of patients, measures the distance patients can walk in 6 min (23). The predicted value of 6MWD reference equation was calculated in reference to the calculation formula; Men: 6MWD = (7.57 × height cm)–(5.02 × age) – (1.76 × weight kg)– 309 m. Women: 6MWD = (2.11 × height cm) – (2.29 × weight kg) – (5.78 × age) + 667 m (24). The CAT was used to assess and monitor the condition of patients with COPD. The CAT consists of 8 questions, the answer to each being scored on a scale of 0 to 5 (25), a higher score indicating a more severe impact of COPD. The CAT was conducted in a face-to-face interview.
Taste sensitivity
Taste sensitivity was evaluated using filter-paper discs (the filter-paper disc method; Taste disc, Sanwa Chemical Laboratory, Nagoya, Japan). This method was created for evaluating taste alteration in the clinical phase (26). Taste recognition thresholds for 4 basic tastes (salty, sweet, sour and bitter) were evaluated using solutions of sodium chloride (NaCl), sucrose, tartaric acid, and quinine hydrochloride. There were 5 concentrations of each solution; concentration one was the lowest and 5 was the highest. The concentrations were 0.300, 1.250, 5.000, 10.000 and 20.000% w/v for NaCl (saltiness), 0.300, 2.500, 10.000, 20.000 and 80.000% w/v for sucrose (sweetness), 0.020, 0.200, 2.000, 4.000 and 8.000% w/v for tartaric acid (sourness), and 0.001, 0.002, 0.100, 0.500 and 4.000% w/v for quinine HCl (bitterness). A droplet of each concentration was deposited on a paper disc, 5 mm in diameter, which was then placed on the tip of the tongue (innervated by the chorda tympani), the posterior tongue (the glossopharyngeal nerve area) and the soft palate (the greater superficial petrosal nerve area) of the subjects for 2 s (26). The taste test started with concentration 1, and gradually increasing to concentration 5 (26). Subjects chose answers from the “basic taste index table”, which listed salty, sweet, sour, bitter, unidentifiable taste, or no taste. Subjects gargle with water in order to avoid the effect of different tastes when the taste was changed (26). The tip of the tongue was always tested first, followed by the posterior tongue and then the soft palate. Quinine hydrochloride was tasted last to avoid the persistence of a strong taste that is distasteful to some subjects (26). The recognition thresholds for basic tastes were determined by their answers. This taste test was carried out at approximately 10.00 h. No patients had any oral intake for at least 1 h before the taste test to avoid the influence of food and drink. The 6MWD, CAT, BMI, FMI, FFMI measurement and the taste test were administered on 2 occasions, once at the beginning and once at the end of the PR programme.
Data analysis
Statistical analysis was performed using Wilcoxon signed-rank test for the values of 6MWD, 6MWD reference equation, 6MWD as % predicted, CAT scores, BMI, FMI, FFMI and taste recognition thresholds at the beginning and end of the PR programme. The results are represented as means (SD). p-values of < 0.05 were considered statistically significant.
RESULTS
All subjects completed the 4-week PR programme. The main clinical characteristics, including means and SD ranges for characteristics of the study population, are shown in Table I.
The mean age of the subjects was 72.4 (SD 8.6) years. Twelve subjects were taking oral medication that might have interfered with taste. All patients took inhalation medication. None of the subjects were current smokers and 8 were ex-smokers. The subjects’ mean BMI was 24.0 kg/m2 (SD 7.9), mean FMI 28.3 kg/m2 (SD 1.5), mean FFMI 16.3 kg/m2 (SD 6.1), mean FVC 2.50 l (SD 0.85), mean FEV1 1.37 l (SD 0.69), and mean FEV1 predicted 67.46% (SD 27.93). FEV1 predicted did not alter significantly (p = 0.13). Mean percent FEV1/FVC was 53.96 (SD 15.42). Five subjects had mild COPD (22.7% of all subjects), 7 had moderate COPD (31.8% of all subjects) and 3 had severe COPD (13.6% of all subjects).
|
Table I. Characteristics of the study subjects (n = 22) |
|||
|
Before |
After |
p-value |
|
|
BMI, kg/m2, mean (SD) |
24.0 (7.9) |
23.5 (6.3) |
0.26 |
|
FMI, %, mean (SD) |
28.3 (11.5) |
28.4 (11.4) |
0.95 |
|
FFMI, %, mean (SD) |
16.3 (6.1) |
16.5 (2.9) |
0.50 |
|
FVC, l, mean (SD) |
2.59 (0.85) |
2.59 (0.82) |
0.96 |
|
FEV1, l, mean (SD) |
1.37 (0.69) |
1.41 (0.69) |
0.19 |
|
FEV1 as % predicted, %, mean (SD) |
67.46 (27.93) |
71.52 (27.01) |
0.13 |
|
FEV1/FVC, mean (SD) |
53.96 (15.42) |
55.07 (17.62) |
0.40 |
|
Medications that alter taste, n (%) |
12 (54.5) |
12 (54.5) |
1.00 |
|
6MWD, m, mean (SD) |
156.7 (134.9) |
239.4 (145.4) |
< 0.01* |
|
6MWD reference equation, m, mean (SD) |
448.8 (60.6) |
452.5 (59.3) |
0.09 |
|
6MWD as % predicted, %, mean (SD) |
37.6 (29.6) |
53.3 (35.1) |
< 0.01* |
|
Ex-smoker, n (%) |
8 (36.4) |
8 (36.4) |
1.00 |
|
CAT scores, mean (SD), median (IQR) |
15.1 (10.5), 14.5 (7.0–25.3) |
11.5 (8.5), 8.5 (6.3–17.0) |
< 0.01* |
|
*Significant difference between the beginning and end of the PR programme (Wilcoxon signed-rank test, p < 0.05). SD: standard deviation; BMI: body mass index; FMI: fat mass index; FFMI: fat-free mass index; FVC: forced vital capacity; FEV1: forced expiratory volume in 1 s; 6MWD: 6-min walking distance; CAT: chronic obstructive pulmonary disease assessment test; IQR: interquartile range. |
|||
The mean value of 6MWD at the end of the PR programme increased significantly compared with at the beginning; 156.7 m (SD 134.5) to 239.4 m (SD 145.4), respectively (p < 0.01). The mean value of 6MWD reference equation at the end of the PR programme did not increase significantly; 448.8 m (SD 60.6) to 452.5 m (SD 59.3). The mean value of 6MWD predicted at the end of the programme increased significantly compared with the beginning of the programme; 37.6% (SD 29.6) to 53.3% (SD 35.1), respectively (p < 0.01). The mean and median values of the CAT scores at the end of the PR programme, 15.1 (SD 10.5) and 8.5 (IQR 6.3–17.0), was significantly lower than at the beginning; 11.5 (SD 8.5) and 14.5 (IQR 7.0–25.3), (p < 0.01). The mean value of BMI was not significantly altered, decreasing from 24.0 kg/m2 (SD 7.9) at the beginning of the programme to 23.5 kg/m2 (SD 6.3) (p = 0.26) at the end. The mean value of FMI was not significantly altered, increasing from 28.3% (SD 11.5) at the beginning of the programme to 28.4% (SD 11.4) at the end (p = 0.95). The mean value of FFMI was not significantly altered, increasing from 16.3 (SD 6.1) at the beginning of the programme to 16.5 (SD 2.9) at the end (p = 0.50). The mean values of FVC, FEV1, FEV1 as % predicted, and FEV1/FVC did not significantly improve (p = 0.96, p = 0.19, p = 0.13, and p = 0.40, respectively).
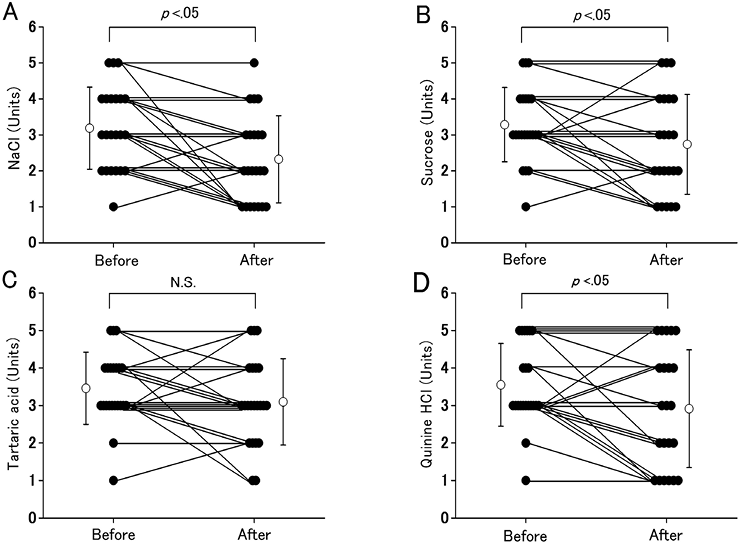
Alterations in taste recognition thresholds are shown in Fig. 1. The mean and median values of salty recognition thresholds at the end of the PR programme was significantly lower than at the beginning, decreasing from 3.2 (SD 1.1) and 3.0 (IQR 2.0–4.0) to 2.3 (SD 1.2) and 2.0 (IQR 1.0–3.0) units (p < 0.01). The mean and median values of sweet recognition thresholds at the end of the programme was significantly lower than at the beginning, decreasing from 3.3 (SD 1.0) and 3.0 (IQR 3.0-4.0) to 2.7 (SD 1.4) and 2.5 (IQR 2.0–4.0) units (p = 0.04). The mean and median values of sour recognition thresholds at the end of the programme was not significantly altered compared with at the beginning, decreasing from 3.4 (SD 1.0) and 3.0 (IQR 3.0–4.0) to 3.1 (SD 1.2) and 3.0 (IQR 2.3–4.0) units (p = 0.15). The mean and median values of bitter recognition thresholds at the end of the PR programme was significantly lower than at the beginning, decreasing from 3.5 (SD 1.1) and 3.0 (IQR 3.0–4.8) to 2.9 (SD 1.6) and 3.0 (IQR 1.3–4.0) units (p = 0.03).
Fig. 1. Alteration in taste recognition thresholds for sodium chloride (NaCl), sucrose, tartaric acid and quinine hydrochloride (quinine HCl) in patients with chronic obstructive pulmonary disease (COPD) between the beginning and end of the pulmonary rehabilitation (PR) programme. (A) Alterations in NaCl recognition thresholds. (B) Alterations in sucrose recognition thresholds. (C) Alterations in tartaric acid recognition thresholds. (D) Alterations in quinine HCl recognition thresholds. Vertical axes show taste recognition thresholds for 4 taste stimuli (NaCl, sucrose, tartaric acid and quinine HCl). Closed circles represent individual data for each taste stimulus. Open circles are means (standard deviations) for all of the patients with COPD. N.S.: no significance.
DISCUSSION
The present study focused on alteration of taste perception by comparing the taste recognition threshold at the beginning of the PR programme with that at the end of the programme.
In some earlier studies, in which patients with COPD participated in maximal incremental cycle ergometer exercise or in a standardized inpatient exercise training programme consisting of daily submaximal cycle ergometer riding, treadmill walking, weight training and gymnastics, weight increased significantly, as did exercise tolerance (16, 17). In the present study, the mean value of BMI, FMI, FFMI in patients with COPD at the end of the PR programme was not significantly increased compared with that at the beginning of the programme. This may be because the period of administration of exercise was short. Another possible reason is that we recruited patients with normal weight; if the patients were thinner we might have observed an increase in body weight.
There was a significantly increased mean value of 6MWD at the end of the PR programme compared with that at the beginning of the programme. This result suggests that after the PR programme patients with COPD exhibited improved exercise tolerance.
There was a significantly decreased mean value of the CAT scores at the end of the PR programme compared with the value of the beginning of the programme. This suggests that the condition of patients with COPD was improved.
Three taste thresholds (salty, sweet and bitter) were significantly lower at the end of the PR programme than at the beginning. An earlier study reported that bitter recognition thresholds were significantly higher for underweight subjects with COPD than for normal-weight subjects (27). There was also a statistical correlation between low body weight and morbidity and mortality. The death rate after weight loss in patients with COPD was significantly higher than in patients with COPD without weight loss (6). Although the taste recognition threshold and BMI did not correlate in this study, improvement in taste sensitivity may have led to increased appetite, which may have prevented exacerbation of COPD.
An earlier study found that the relationship between bitter taste and smoking was relative to the period and amount of smoking. That study concluded that the duration of smoking might be an important criterion (28). The present study found no significant differences between taste recognition thresholds of ex-smokers and non-smokers.
Salty and sour taste stimulations modulate taste-cell function by direct entry of calcium (Na+) and hydrogen (H+) ions through specialized membrane channels on the surface of the cell. The identity of the salt receptor remains speculative and highly controversial (29). Recent genetic and functional studies have shown that the sour receptor is a member of the transient receptor potential (TRP) ion-channel family (30). Both sweet and bitter compounds bind to G-protein-coupled receptors (GPCRs), which weave in and out of the cell membrane. They change the conformation of cells and trigger the action of a guanosine triphosphate-binding protein in the cell membrane (31). The results of this study represent alterations in salty, sweet and bitter recognition thresholds. These results suggest that taste perception might be improved by the PR programme regardless of the structures and mechanism of taste receptors.
This study has 3 limitations. First, it lacks comparison of the results of the taste test in patients with COPD with those of the taste test in healthy elderly people. Secondly, it was not a randomized trial because such a test was not ethically justified in patients with COPD who were willing to participate in the PR programme. Thirdly, since we recruited patients with normal weight, it might be difficult to apply this result to patients with COPD with emaciation.
In conclusion, this study suggests that a PR programme may improve taste sensitivity in patients with COPD, contributing to avoiding weight loss and improving the prognosis for patients with COPD.
AcknowledgEments
The authors thank Thomas Mandeville for assistance with editing. This study was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (JSPS KAKENHI grant numbers 23659375, 24300187, 24659397, and 25 • 7166), Research Grant for Longevity Sciences from the Ministry of Health, Labor and Welfare (H22-Junkanki-shi-Ippan-001), and Research Funding for Longevity Sciences (22-2) from the National Center for Geriatrics and Gerontology (NCGG).
The authors declare no conflicts of interest.
REFERENCES
