Won-Seok Kim, MD1, Se Hee Jung, MD, PhD2, Min Kyun Oh, MD1, Yu Sun Min, MD1, Jong Youb Lim, MD1 and Nam-Jong Paik, MD, PhD1
From the 1Department of Rehabilitation Medicine, Seoul National University College of Medicine, Seoul National University Bundang Hospital, Seongnam-si, Gyeonggi-do and 2Department of Rehabilitation Medicine, Seoul National University Boramae Medical Center, Seoul, South Korea
OBJECTIVE: To investigate the safety, feasibility and preliminary efficacy of low-frequency repetitive transcranial magnetic stimulation (rTMS) over the cerebellum in ataxic patients with acute posterior circulation stroke.
DESIGN: Randomized, double-blind, sham-controlled pilot study.
Patients: Thirty-two ataxic patients with posterior circulation stroke were randomized to real (n = 22) and sham (n = 10) rTMS groups.
METHODS: Patients received 5 15-min sessions of 1 Hz cerebellar rTMS over 5 consecutive days. Compliance and adverse events for the rTMS sessions were checked. The 10-m walk test (10MWT) and Berg Balance Scale (BBS) were completed before rTMS, immediately and 1 month after the last rTMS session.
RESULTS: Compliance with the rTMS was 100% and no adverse events were reported in either group. 10MWT and BBS of real rTMS group improved significantly (p < 0.01). Percentage changes immediately after the last rTMS session for time and steps in the 10MWT and BBS in the real vs sham group were: –16.7 ± 35.1% vs –8.4 ± 72.5%, –8.5 ± 23.0% vs –0.3 ± 28.4% and 46.4 ± 100.2% vs 36.6 ± 71.6%, respectively.
CONCLUSION: This study demonstrated that 1 Hz rTMS over the cerebellum is safe, feasible and may have a beneficial effect in ataxic patients with posterior circulation stroke.
Key words: transcranial magnetic stimulation; cerebellum; posterior circulation brain infarction; ataxia; stroke; randomized controlled trial; safety; feasibility studies.
J Rehabil Med 2014; 46: 00–00
Correspondence address: Nam-Jong Paik, Department of Rehabilitation Medicine, Seoul National University College of Medicine, Seoul National University Bundang Hospital,166 Gumi-ro, Bundang-gu, Seongnam-si, Gyeonggi-do, 463-707, South Korea. E-mail: njpaik@snu.ac.kr
Accepted Jan 8, 2014; Epub ahead of print Mar 24, 2014
INTRODUCTION
Ataxia is a common impairment after posterior circulation stroke (PCS) involving the cerebellum or brain stem, which leads to restrictions of mobility and activities of daily living (1, 2). In these patients, recovery from ataxia is as important as recovery from weakness for obtaining independent mobility (3). Various strategies have been suggested as treatments for ataxia after stroke, but the evidence for their effectiveness is limited (3).
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive brain stimulation method. Its efficacy in enhancing recovery has been demonstrated in various impairments after stroke (4, 5). The stimulation site of rTMS differs according to the impairment for which post-rTMS recovery is expected (5). Ataxia after PCS has been thought to be associated with damages to the cerebellum or cortico-ponto-cerebellar projections (cerebellar afferent pathways) (6, 7). Therefore, the cerebellum has been considered as a potential stimulation site for rTMS for enhancing recovery from ataxia (8, 9).
Cerebellar stimulation in healthy people can modulate primary motor cortex excitability by changing cerebello-cerebral inhibition (10, 11). Cerebello-cerebral inhibition alteration by cerebellar rTMS is expected to modulate the abnormalities in corticomotor excitability in ataxic patients with brain lesions (6, 12–14), which could lead to recovery from ataxia. A study showing that changes in cerebellar excitability are associated with human locomotor adaptive learning also suggests a possible role of cerebellar rTMS as a therapeutic tool in patients with stroke (15).
Previous studies have reported that low-frequency rTMS over the cerebellum can improve walking ability in patients with spinocerebellar degeneration (8, 9). It was also reported that 1Hz rTMS targeted at the lateral cerebellum could induce a faster response in the ipsilateral upper extremity in early-stage Parkinson’s disease (16). However, no study has been conducted to determine the effect of cerebellar rTMS on patients with ataxia after PCS.
Therefore, we designed this randomized, double-blind, sham-controlled pilot study to primarily investigate whether low-frequency (1Hz) rTMS over the cerebellum for 5 days is safe and feasible in ataxic patients with acute PCS. A further objective was to examine the preliminary efficacy of low-frequency cerebellar rTMS as an add-on therapeutic modality to inpatient conventional rehabilitation in ataxic patients after PCS.
PATIENTS AND METHODS
Patients
Patients were recruited from May 2009 to December 2011. Patients were eligible for inclusion in the study if they had acute (< 3 months), first-ever ischaemic cerebellar or brain stem stroke with symptoms of ataxia. Exclusion criteria were: younger than 18 years of age; ischaemic stroke in multiple vascular territories; severe weakness or ataxia that interfered with functional evaluation; increased intracranial pressure; contraindications to rTMS, such as implanted pacemakers and history of seizure; and inability to provide written informed consent. All subjects received detailed information about the study and provided their written consent. The research protocol was approved by the local institutional review board and was conducted in accordance with the regulatory standards of Good Clinical Practice and the Declaration of Helsinki (World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects, 2008).
Of the 41 eligible subjects, 32 were enrolled and randomly assigned into real (n = 22) and sham rTMS (n = 10) groups (Fig. 1). At the 1-month follow-up, 2 patients in the real rTMS group and 4 in the sham rTMS group were lost to follow-up for reasons not related to the intervention (Fig. 1). There were no significant differences between the 2 groups in terms of demographic variables, stroke subtype, onset time of stroke and baseline balance and gait function (Table I).
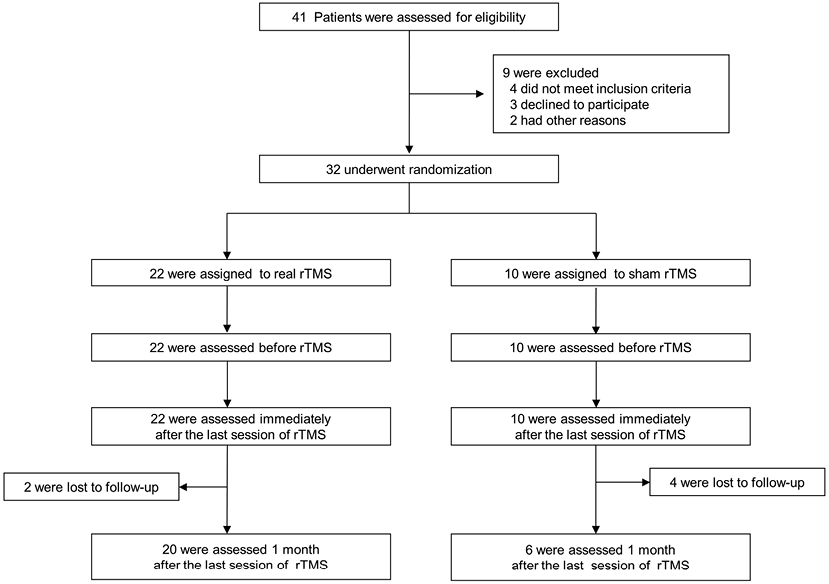
|
Table I. Baseline characteristics of patients |
|||||||
|
Variable |
Per-protocol analysis |
Intention-to-treat analysis |
|||||
|
Sham rTMS (n = 6) |
Real rTMS (n = 20) |
p-value |
Sham rTMS (n = 10) |
Real rTMS (n = 22) |
p-value |
||
|
Age, years, mean (SD) |
66.7 (11.4) |
66.7 (7.7) |
0.997a |
|
64.8 (11.7) |
67.4 (7.8) |
0.531a |
|
Sex, n (%) |
|||||||
|
Male |
3 (50.0) |
10 (50.0) |
1.000b |
|
6 (60.0) |
11 (50.0) |
0.712b |
|
Female |
3 (50.0) |
10 (50.0) |
4 (40.0) |
11 (50.0) |
|||
|
Stroke lesion, n (%) |
|||||||
|
Cerebellum |
2 (33.3) |
5 (25.0) |
0.799b |
|
4 (40.0) |
5 (22.7) |
0.619b |
|
Pons |
3 (50.0) |
9 (45.0) |
4 (40.0) |
11 (50.0) |
|||
|
Medulla |
1 (16.7) |
6 (30.0) |
2 (20.0) |
6 (27.3) |
|||
|
Time from stroke to real or sham rTMS (days), mean (SD) |
14.0 (4.9) |
16.8 (13.4) |
0.624a |
15.1 (5.1) |
16.2 (13.0) |
0.581a |
|
|
10-m time, sd, mean (SD) |
68.6 (81.4) |
45.8 (21.7) |
0.855c |
|
58.6 (67.4) |
45.7 (20.7) |
0.515c |
|
10-m stepse, mean (SD) |
41.2 (18.3) |
36.1 (13.8) |
0.464c |
38.1 (17.7) |
37.6 (14.3) |
0.871c |
|
|
BBS, mean (SD) |
25.0 (17.3) |
25.1 (13.9) |
0.879c |
27.6 (16.6) |
24.6 (13.6) |
0.415c |
|
|
aStudent’s t-test for independent samples. bχ2 test. cMann-Whitney U test dTime in 10-m walk test. eNumber of steps in 10-m walk test. rTMS: repetitive transcranial magnetic stimulation; BBS: Berg Balance Scale. |
|||||||
Experimental design
A double-blind, randomized, sham-controlled trial was performed. Patients were randomized in a 2-to-1 ratio to receive either real or sham rTMS over the cerebellum. Unequal randomization was used in order to increase the possibility of gathering more information in the real cerebellar rTMS group, such as the data for compliance and adverse events (17). A randomization sequence was generated by a computer and concealed using opaque envelopes. This procedure was performed by the principal investigator (N-J), who was not involved in the selection, intervention and assessment of patients. Both patients and assessors were blinded to group allocation. Five 15-min sessions of real or sham rTMS were applied to patients for 5 consecutive days. Adverse events were checked during and after each rTMS session by the investigators (JYL, YSM). Assessment was performed before the rTMS, immediately and 1 month after the last session of rTMS (Fig. 2) by physical therapists blinded to the group of rTMS intervention. Patients were not informed of the group assignment. Patients were not allowed to discuss the rTMS intervention they received with other patients or physical therapists. All participants received conventional rehabilitation service, such as gait and balance training during admission.
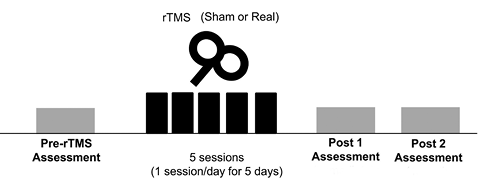
Fig. 2. Experimental design. Patients received 5 15-min daily real or sham repetitive transcranial magnetic stimulations (rTMS) over a period of 5 days. Measurements were performed prior to treatment (Pre-rTMS), immediately (post 1) and 1 month (post 2) after the last session of rTMS.
Intervention
The patient was seated comfortably in a chair during the rTMS session. Before the cerebellar rTMS, the resting motor threshold (RMT) for the abductor pollicis brevis muscle in the non-ataxic side was measured over the M1 of the hemisphere ipsilateral to the ataxic side. The minimum stimulation intensity needed to evoke a response of at least 50 µV in at least 5 of 10 consecutive stimulations was determined as a RMT (18). Cerebellar rTMS was performed through a 75 mm-diameter figure-of-8 coil powered by MagPro® (Medtronic, Minneapolis, MN, USA). The coil was placed 2 cm below the inion and 2 cm lateral to the midline on the cerebellar hemisphere ipsilateral to the ataxic side, with the handle pointing superiorly, targeting the posterior cerebellar lobe (10, 19). Each patient received 5 sessions of cerebellar rTMS over 5 consecutive days. Stimulation in each session was applied at a frequency of 1 Hz and an intensity of 100% of the patient’s RMT for 15 min, achieving 900 stimuli in total per session. For the sham rTMS, the coil was placed perpendicular to the scalp with the same parameters of stimulation to minimize current flow into the skull (20).
Outcome measurements
For the feasibility outcome, compliance with the interventions was selected. Compliance was defined as “(number of planned sessions of treatment/number of attended sessions)×100 (%)” (21).
We investigated the incidence of the adverse events during and after each rTMS session as the safety outcome including nausea, local pain at stimulation site, neck pain, muscular neck stiffness, headache, sleepiness, psychotic symptom and seizure.
The subjects were functionally assessed at baseline and immediately and 1-month after the last session of rTMS. Time and steps in the 10-m walk test (10MWT) and Berg Balance Scale (BBS) were selected. The time and number of steps were assessed as subjects walked 10 m at a self-selected speed with or without a gait aid. This is a valid and reliable measure for walking ability in stroke patients (22, 23). The BBS is a 14-item scale widely used to evaluate balance in stroke patients (24). The total score ranges from 0 to 56, with a higher score indicating better balance function.
Statistical analyses
Although this is a pilot study and the primary objective of the study is to investigate feasibility and safety, we calculated a sample size for the efficacy of the low-frequency rTMS over the cerebellum in PCS. Thirty-two patients were needed to detect a difference of 3.75 s in time of 10MWT between the 2 groups (with β = 0.20 and α = 0.05) based on previously published data in patients with spinocerebellar degeneration (8), taking into account the 10% drop-out rate and 2-to-1 random allocation (25). Because we applied a substantially higher number of magnetic stimuli than that of the previous study (8), the higher effect size was expected.
Continuous variables are presented as means (standard deviations; SDs). Categorical variables are presented as frequencies (percentages). To compare the baseline characteristics between the 2 groups, Student’s t-test or the Mann-Whitney U test (for non-normally distributed data) was used for continuous variables and χ2 test was used for categorical variables. An uncorrected 2-tailed p < 0.05 was considered statistically significant.
Both per-protocol and intention-to-treat analyses were used in analysing functional outcomes (time and steps in 10MWT, and BBS). For the per-protocol analysis, patients who did not complete the entire study protocol (e.g. follow-up loss) were excluded from the analysis. In comparison, intention-to-treat analysis included every subject who was randomized and missing data at the follow-up assessment was imputed by using the last observation carried forward approach. For the analysis of functional outcomes, Mann-Whitney U test and Friedman’s test were chosen over parametric tests due to the small sample size and the pilot nature of this study. Comparisons of score at each time-point between the 2 groups were performed by Mann-Whitney U test with a 2-tailed Bonferroni corrected p < 0.017 to adjust the type I error due to multiple comparisons. Changes in functional outcomes within each group were analysed with Friedman’s test for multiple time-points. Wilcoxon signed-rank test with Bonferroni correction was applied as a post-hoc test only when Friedman’s test revealed overall significant differences (p < 0.05) and a 2-tailed p < 0.017 was considered statistically significant.
Standardized effect sizes for percentage changes between the baseline score and score immediately after the last rTMS session of functional outcome measures in intention-to-treat analysis between the 2 groups were calculated. Because there were losses to follow-up at 1 month after the last session of rTMS (Fig. 1), the effect sizes for functional outcomes at 1 month were not calculated. Standardized effect sizes were calculated with the following equation: standardized effect size = (differences in mean percentage changes in outcomes between real and sham rTMS groups)/(pooled standard deviations) (26). Statistical analysis was performed using the PASW statistical package (SPSS version 18.0, SPSS, Chicago, IL, USA).
RESULTS
Feasibility and safety outcomes
For the feasibility outcome, compliance with the rTMS was 100% in the both real and sham rTMS groups. Adverse events were not reported during and after the rTMS session in both real and sham rTMS groups.
Functional outcomes
All functional outcome measures showed no differences between the real and sham rTMS groups at every time-point by Mann-Whitney U test in both per-protocol and intention-to-treat analyses.
All functional outcome measures of real rTMS group in both per-protocol and intention-to-treat analyses improved significantly (Table II). Post-hoc analysis with Wilcoxon signed-rank test showed that time and number of steps in 10MWT improved significantly at 1 month after real rTMS compared with baseline, but the improvement in time and number of steps immediately after the rTMS was not significant (Table II). In the real rTMS group, BBS improved significantly immediately after the last rTMS session (p = 0.004) and there was further improvement until 1 month after the last rTMS session (p = 0.001) (Table II).
In the sham rTMS group, Friedman’s test showed that the trends of improvement in BBS are significant in both per-protocol (p = 0.003) and intention-to-treat analyses (p = 0.002) (Table II). Post-hoc analysis with Wilcoxon signed-rank test in intention-to-treat analysis showed that BBS after the last session of sham rTMS significantly improved (p = 0.008) and that improvement was maintained until 1 month after the last session (Table II). In the sham rTMS group, the trend of decrease in time in 10MWT was significant (p = 0.016) only in the per-protocol analysis (Table II).
|
Table II. Functional outcomes at baseline, immediately after the last rTMS session (post 1) and 1 month after the last rTMS session (post 2) |
|||||||||||||
|
Outcome |
Sham rTMS |
Real rTMS |
|||||||||||
|
Baseline Mean (SD) |
Post 1 Mean (SD) |
Post 2 Mean (SD) |
p-valuea |
Baseline Mean (SD) |
Post 1 Mean (SD) |
Post 2 Mean (SD) |
p-valuea |
||||||
|
Per-protocol analysis (n = 6 in sham rTMS group and n = 20 in real rTMS group) |
|||||||||||||
|
10-m time, sd |
68.6 (81.4) |
46.6 (65.0) |
27.6 (27.5) |
0.016* |
45.8 (21.7) |
39.5 (25.6) |
26.0 (20.0)b |
0.001* |
|||||
|
10-m stepse |
41.2 (18.3) |
32.8 (11.9) |
26.8 (11.7) |
0.084 |
36.1 (13.8) |
33.3 (12.9) |
26.9 (8.0)b |
0.004* |
|||||
|
BBS |
25.0 (17.3) |
31.5 (15.9) |
42.2 (9.8) |
0.003* |
25.1 (13.9) |
31.6 (16.0)b |
39.5 (13.3)b,c |
< 0.001* |
|||||
|
Intention-to-treat analysis (n = 10 in sham rTMS group and n = 22 in real rTMS group) |
|||||||||||||
|
10-m time, sd |
58.6 (67.4) |
46.8 (56.7) |
35.3 (37.2) |
0.062 |
45.7 (20.7) |
38.3 (24.7) |
26.0 (19.0)b |
< 0.001* |
|||||
|
10-m stepse |
38.1 (17.7) |
35.8 (15.8) |
32.2 (16.8) |
0.549 |
37.6 (14.3) |
33.3 (12.3) |
27.5 (7.9)b |
0.001* |
|||||
|
BBS |
27.6 (16.6) |
32.6 (16.5)b |
39.0 (14.2)b |
0.002* |
24.6 (13.6) |
30.8 (15.8)b |
38.0 (14.0)b,c |
< 0.001* |
|||||
|
*p < 0.05. ap-values by the Friedman’s test within each group. bp < 0.017 by the Wilcoxon signed-rank test with Bonferroni correction (post-hoc test for the Friedman’s test) vs baseline within each group. cp < 0.017 by the Wilcoxon signed-rank test with Bonferroni correction (post-hoc test for the Friedman’s test) vs post 1 within each group. dTime in 10-m walk test. eNumber of steps in 10-m walk test. SD: standard deviation; rTMS: repetitive transcranial magnetic stimulation; BBS: Berg Balance Scale. |
|||||||||||||
Percentage changes immediately after the last rTMS session for the time and steps in 10MWT and BBS in the real vs sham group were: –16.7 ± 35.1% vs –8.4 ± 72.5%, –8.5 ± 23.0% vs –0.3 ± 28.4%, and 46.4 ± 100.2% vs 36.6 ± 71.6%, respectively. Standardized effect sizes for percentage changes in functional outcome measures immediately after the last rTMS session were –0.17 for time in 10MWT, –0.33 for number of steps in 10MWT, and 0.11 for BBS. A power analysis (1-tailed t-test, α = 0.05, β = 0.2) indicated that 429, 115 and 1023 subjects per group may be needed to detect the differences between 2 groups in time and number of steps in 10MWT, and BBS, respectively.
DISCUSSION
This is the first randomized, controlled, double-blind pilot study to investigate the safety, feasibility and preliminary effect of low-frequency rTMS over the cerebellum in ataxic patients with PCS. The results show that 5 sessions of 15 min 1 Hz rTMS over the cerebellar hemisphere ipsilateral to the ataxic side for 5 consecutive days were well tolerated and safe. Overall walking ability, measured by 10MWT, improved significantly only in the active rTMS group using both per-protocol and intention-to-treat analyses (Table II). Percentage changes in improvement in the time and steps in 10MWT immediately after the last rTMS session were 2.0 and 28 times greater in the real rTMS group compared with the sham rTMS group. Immediately after the last rTMS session, standardized effect size was moderate for the number of steps and small for the time in 10MWT and BBS.
Few studies have reported adverse events of cerebellar rTMS (16). Satow et al. (27) reported that there was induced nausea in 2 participants among 8 healthy subjects, lasting 10 min after a 0.9Hz and 900 pulses of rTMS over the right cerebellum. Brighina et al. (28) reported 2 of 17 subjects with migraine reported mild muscular neck stiffness. In this study, 900 stimuli of 1 Hz cerebellar rTMS for 5 consecutive days induced no adverse events including nausea, neck pain or aggravation of neurological deficits. Therefore, the protocol for cerebellar rTMS in this study seems to be safe for use in patients with PCS. Furthermore, in the real rTMS group, there were no drop-outs during the rTMS sessions or 1 month after the last session of the rTMS, therefore the compliance and feasibility of cerebellar rTMS was good (Fig. 1).
Various abnormalities in corticomotor excitability have also been reported in patients with cerebellar lesions (12, 13). The changes in corticomotor excitability in these patients have been associated with changes in cerebello-cerebral inhibition (29). Therefore, modulation of cerebello-cerebral inhibition through cerebellar rTMS (30) can be applied as a therapeutic tool for these patients. We selected low-frequency (1 Hz) rTMS over the cerebellum as a therapeutic modality to improve ataxia in this study. In healthy subjects, low-frequency rTMS on the cerebellum resulted in controversial effects on M1 excitability (10, 31). Furthermore, it is not clear whether the cerebellar rTMS affects the Purkinje cells in the cerebellar cortex or dentate nucleus, each of which plays a different role in cerebello-cerebral inhibition (32). Therefore, the effect of cerebellar rTMS and which stimulation protocol is beneficial for recovery of motor function in patients with cerebellar lesions remain unclear. Previous studies of low-frequency cerebellar rTMS in patients with spinocerebellar degeneration have demonstrated improvement in the 10MWT (8, 9). In addition, low-frequency rTMS of the cerebellum in Parkinson’s disease improved task performance (16), and the decreased cerebellar excitability was associated with better locomotor adaptive learning (15). Based on these results, a low-frequency stimulation protocol was selected in the current study.
According to some previous studies, which have suggested that decreased Purkinje cell excitability in patients with PCS can decrease cerebello-cerebral inhibition and may lead to ataxia (16, 33), the low-frequency cerebellar rTMS could inhibit the excitability of Purkinje cells and further decrease cerebello-cerebral inhibition, which can aggravate the abnormal condition (33). However, we observed that the low-frequency cerebellar rTMS group showed a significant improvement in 10MWT with no harmful effect on balance function measured by BBS, which is consistent with the results of previous studies in patients with spinocerebellar degeneration (8, 9). Percentage changes in the results of the 10MWT immediately after the fifth rTMS session were much higher in the real rTMS group compared with the sham rTMS group.
There are several possible explanations for the discrepancy between the hypothesis suggested by the previous studies (16, 33) and the results of clinical trials in patients including our studies (8, 9). First, because efferent pathways from the cerebellum are connected with both excitatory and inhibitory neurones in the motor cortex, it is difficult to predict whether the modulation of cerebello-cerebral inhibition increases or decreases the excitability of the motor cortex (10, 31, 34). This complexity of cerebello-cerebral connection may make the prediction of clinical outcomes after cerebellar rTMS difficult. However, as we did not include the neurophysiological measurement, such as a paired-pulse TMS, it was difficult to verify the association between the changes in the excitability of the cerebellum or motor cortex and recovery from ataxia. Secondly, cerebellar rTMS can affect the excitability of the dentate nucleus (32). In this situation, low-frequency cerebellar rTMS can decrease the excitability of the dentate nucleus, and this can increase the abnormally decreased cerebello-cerebral inhibition in PCS. Thirdly, our results can be explained by the association between cerebellar excitability and locomotor adaptive learning. Human locomotor adaptive learning is proportional to depression of cerebellar excitability, which may be mediated by long-term depression in Purkinje cells (15). Therefore, it is possible that the depression of cerebellar excitability induced by low-frequency cerebellar rTMS enhances the locomotor adaptative learning during conventional stroke rehabilitation and this may lead to better improvement in walking ability.
This study has several limitations to be considered. First, it is difficult to exclude the possibility of antidromic corticospinal tract activation by cerebellar rTMS, which can suppress the contralateral motor cortex (35, 36). Although we used the intensity of rTMS as a 100% of RMT and handled the coil superiorly in order to minimize this effect, there are no specific suggestions about how to solve this problem (35). Future studies with measurement of brain stem excitability (37) may be helpful in order to exclude the influence of antidromic pyramidal tract activation on changes in motor cortex excitability and recovery from ataxia. Secondly, we did not include the neurophysiological method, such as paired-pulse TMS, which can be helpful for direct verification of the change in cerebellar excitability by low-frequency cerebellar rTMS (15). Thirdly, patients in our study are heterogeneous in the baseline function and anatomical structures involved in PCS (Table I). It has been known that responsiveness to rehabilitation therapy differs according to the stroke severity (38). In addition, it has been suggested that ataxia occurs in medullary infarction by involving the spino-cerebellar pathway, in pontine infarction by involving the olivo-ponto-cerebellar pathway, and in cerebellar infarction by involving the cerebellar cortex or dentate nucleus. Cerebellar rTMS can deliver different effects on each pathway, which may lead to different responsiveness to rTMS. Therefore, the heterogeneities in baseline function and anatomical structures involved might lead to high standard deviations in percentage changes of outcome measures and decrease the statistical power. Fourthly, the estimated sample size based on the previous study with more homogeneous patients group (8) might be too small to detect the effect of the cerebellar rTMS in our study with more heterogeneous patients group. In addition, our hypothesis that more stimuli in our study protocol would result in a greater effect than the previous study (8) might underestimate the sample size. Further research should estimate the sample size based on the results of this study, and include a more homogenous patients group, which may increase the statistical power. Fifthly, the rehabilitation therapy and medications after discharge were not controlled in the participants, which could influence recovery from ataxia. Finally, 6 patients (19% of total participants, 2 in the real rTMS group and 4 in the sham rTMS group) dropped out during follow-up, although the reasons for follow-up loss were not related to the cerebellar rTMS.
In conclusion, this pilot study demonstrated that 1 Hz rTMS over the cerebellum is safe, feasible and may have possible beneficial effects in ataxic patients with PCS. The study results therefore encourage the careful design of a clinical trial with a larger sample size and homogenous patient group in terms of anatomical lesions in order to clarify the therapeutic mechanism and role of cerebellar rTMS in patients with ataxia after PCS.
ACKNOWLEDGEMENTS
This study was supported by a grants from the Korean Health Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (grant number A101901), and the SNUBH Research Fund (grant number 03-2009-004). The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
