Yong Hee Lee, PhD1, Eun Sun Yoon, PhD1, Soo Hyun Park, PhD1, Kevin S. Heffernan, PhD2, Chong Lee, EdD3 and Sae Young Jae, PhD1
From the 1Department of Sport Science, University of Seoul, Seoul, South Korea, 2Department of Exercise Science, Syracuse University, Syracuse and 3Exercise and Wellness Program, Healthy Lifestyles Research Center, Arizona State University, Mesa, AZ, USA
OBJECTIVE: To determine whether arterial stiffness is associated with cognitive function after adjustment for physical fitness in patients with chronic stroke.
METHODS: Cross-sectional analyses were conducted in 102 patients with chronic stroke who participated in an exercise rehabilitation programme. Carotid femoral pulse wave velocity and augmentation index were measured as indices of arterial stiffness and central systolic loading. Cognitive function was assessed with the Mini Mental State Examination. Parameters of physical fitness included the 6-min walk test, flexibility, balance, and muscle strength tests.
RESULTS: Carotid femoral pulse wave velocity was significantly associated with Mini Mental State Examination (r = –0.45, p < 0.01) and parameters of physical fitness (r = –0.45~ –0.55, p < 0.01, all). Mini Mental State Examination was significantly associated with parameters of physical fitness (r = 0.32~0.46, p < 0.01, all). In multivariable linear regression models, carotid femoral pulse wave velocity was inversely associated with Mini Mental State Examination after adjustment for multiple risk factors (beta = –0.33, p = 0.01). However, the association was attenuated and became non-significant after additional adjustment for physical fitness (beta = –0.11, p = 0.39).
CONCLUSION: Arterial stiffness measured by carotid femoral pulse wave velocity is associated with cognitive function in patients with chronic stroke, but not after adjustment for physical fitness. Maintaining appropriate levels of physical fitness may have a favourable effect on both vascular and cognitive function in patients with stroke.
Key words: arterial stiffness; cognitive function; physical fitness; stroke.
J Rehabil Med 2014: 46: 00–00
Correspondence address: Sae Young Jae, Health and Integrative Physiology Laboratory, Department of Sport Science, University of Seoul, 90 Jeonnong-dong, Dongdaemun-gu, Seoul 130-743, South Korea. E-mail: syjae@uos.ac.kr
Accepted Dec 10, 2013; Epub ahead of print Feb 28, 2014
INTRODUCTION
Increased arterial stiffness is an independent predictor for cardiovascular diseases and all-cause mortality (1). Increased arterial stiffness with subsequent increases in central systolic loading and haemodynamic pulsatility is a predictor for stroke (2) and is associated with microvascular damage to the brain (3). The brain is a high-flow organ that may be sensitive to excessive pressure and flow pulsatility (4, 5). The American Heart Association and the American Stroke Association have acknowledged the importance of vascular dysfunction as a significant factor governing cognitive impairment with ageing and disease (6). Arterial stiffness and pulsatile central haemodynamics have both been shown to be associated with reduced cognitive function in older adults (7–14). This is significant, as cognition is the most important determinant of overall health status, quality of life and functional ability with advancing age and disease (15–17). Cognitive function of patients with stroke is significantly attenuated compared with age-matched healthy peers (18), and is associated with microvascular damage in the brain (19). Therefore, it is possible that stroke patients have damage to their cerebral-vasculature related to increased arterial stiffness, and this may further contribute to decreased cognitive function.
Increased cardiorespiratory fitness is associated with reduced cardiovascular risk factors and mortality (20). In addition, a high level of physical fitness is associated with decreased arterial stiffness and central systolic load (21) as well as higher cognitive function in adults with neurological disorders (22). Therefore, considering the inter-relation between arterial stiffness, cognitive function and physical fitness, it is possible that high physical fitness might favourably affect the relationship between increased arterial stiffness and decreased cognitive function. However, no study has been conducted on such a possibility. We tested the hypothesis that the association between arterial stiffness and cognitive function would be mediated by physical fitness in chronic stroke patients.
METHODS
Subjects
Patients with time since onset of stroke > 1 year (n = 133) were recruited from the Hanwoori Disabilities Rehabilitation Center (Seoul, Korea) (Fig. 1). Exclusion criteria were: those without significant symptoms of hemiplegia (transient ischaemic attack) (n = 5); those with orthopaedic disease in the lumbo-pelvic-hip complex and/or limb (arthritis) (n = 4); those who underwent cardiac surgery (artificial heart, severe arrhythmia) (n = 4); those with a wheelchair (n = 6); and those who were incapable of following instructions/incapable of communicating (n = 2). Subjects were also excluded if they could not complete the 6-min walk test (6MWT) (n = 3); if they needed to stop during the 6MWT (n = 3); or if they walked too slowly (n = 4). Thus, 102 subjects met all requirements for inclusion in this study. All experimental procedures and protocols were approved by the Public Institutional Review Board (PIRB12-060-02). Each subject provided written informed consent.
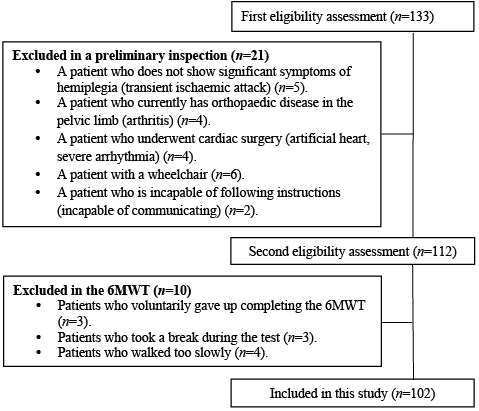
Fig. 1. Subjects’ flow diagram. 6MWT: 6-min walk-test.
Experimental measurements
Body anthropometric variables, such as height (cm), weight (kg), and body mass index (BMI) (kg/m2) were measured using multi-frequency impedance analysis and an extensometer (Inbody 3.0, Biospace, Seoul, South Korea). After 10 min of quiet rest in the supine position, brachial arterial blood pressure was obtained using an automatic monitor (UA-711, Takeda Medical, Tokyo, Japan). Pulse pressure (PP) was calculated as systolic blood pressure minus diastolic blood pressure. Arterial stiffness was measured in accordance with the Clinical Application of Arterial Stiffness, Task Force III guideline (23). Carotid-femoral pulse wave velocity (PWV) was measured by dividing the distance (D) between the carotid artery and femoral artery by the time difference (Δt) between the 2 sites (PWV = D/Δt m/s). D between the carotid and femoral arteries was obtained with the use of a measuring tape, and this distance was subtracted from the distance between the carotid artery and the supra-sternal notch.
Central/aortic blood pressure was automatically analysed through the pulse wave detected at the radial artery using SphygmoCor (SphygmoCor device, AtCor Medical, Sydney, Australia) with the generalized radial-aortic transfer function. Augmentation index (AIx) was derived from the aortic pressure waveform and automatically adjusted to a heart rate of 75 bpm (AIx75). Vascular and blood pressure (BP) measurements were taken between 09.00 h and 12.00 h after subjects had abstained from caffeinated beverages and food for > 3 h.
For measurement of cognitive function, the Korean version of the Mini-Mental Status Examination was used (MMSE-K), as originally developed by Folstein et al. (24).
To measure physical fitness, various tasks were performed to appraise cardiorespiratory fitness, muscular strength, flexibility and balance. For cardiorespiratory fitness, the 6MWT was used following guidelines suggested by Crapo et al. (25). A 30-m track, marked every 2 m, was used and to measure the distance covered in the 6MWT (26). For muscular strength, handgrip strength of the stroke-unaffected side was measured using a grip strength dynamometer (TKK 5401, Takei Scientific Instruments, Niigata, Japan). For this test, the patient was required to sit in a chair without armrests and activate the elbow flexors with the elbow maintained at a 90º angle. As suggested by Rikli & Jones (27), flexibility was measured by the chair sit and reach test (cm). For this method, the subjects sat on a chair with their entire sole of the affected side touching the floor and extended their unaffected leg with the heel touching the floor. The subjects then bent forward and extended their hands with their ankle of the extended leg dorsiflexed, and the extended length of the hands was measured. If the measurement (cm) was above the toe, it was marked as negative (–); if it was below the toe, it was marked as positive (+). For balance measurement, the functional reach test (cm) was performed as described by Katz-Leurer et al. (28). The starting position in the Functional Reach Test is as follows: a patient is required to stand and hold his/her elbow extended with 90º of shoulder flexion maintaining one arm in a horizontal position. They then lean forward, stretching out the fingers and are asked to reach forward as far as they can. The difference between the length of a person’s outstretched arm and the maximal reach forward at the end position of the metacarpophalangeal (MCP) joint is measured with a measuring stick mounted on the wall. Muscular strength, flexibility, and balance were measured twice, and their mean values reported.
Statistics
All data are presented as means and standard deviations (SD). The normal distribution of the variables was tested using the Kolmogorov-Smirnov test. Pearson correlations and multiple regression models were used to describe the associations among arterial stiffness, cognitive function, and physical fitness. A z-score was used to create a global measure of physical fitness from the separate measures of cardiorespiratory fitness, muscular strength, flexibility and balance. Separate regression models were used to investigate the association between arterial stiffness and cognitive function with adjustment for multiple risk factors (age, BMI, duration of stroke, education level, systolic blood pressure, cigarette smoking and medications) and additional adjustment for physical fitness. All statistical procedures were performed with SPSS software (version 19; SPSS Inc., Chicago, IL, USA) and all p-values are two-sided, with an α-level of 0.05.
RESULTS
Participant characteristics are shown in Table I. The inter- correlations among arterial stiffness, cognitive function and physical function are described in Table II. There was a significant inverse correlation between carotid-femoral PWV and cognitive function in Fig. 2 (r = –0.45, p < 0.01). There was also a significant inverse correlation between carotid-femoral PWV and global physical fitness score (r = –0.66, p < 0.01). However, AIx did not show any significant correlation with cognitive function (r = –0.01, p = 0.92), and showed a negative correlation only with muscular strength (r = –0.38, p < 0.01). MMSE score was associated with each component of physical fitness as well as the global z-score (p < 0.05). As shown in Table III, a significant correlation was found between carotid-femoral PWV and cognitive function (β = –0.33, p = 0.001) after adjustment for age, BMI, duration of stroke, education level, systolic blood pressure, cigarette smoking and medications (Model 1). However, the association was attenuated and became non-significant after additional adjustment for physical fitness (β = –0.11, p = 0.39) (Model 2).
|
Table I. Subjects’ characteristics (n = 102) |
|
|
Characteristics |
|
|
Age, years, mean (SD) |
61 (9) |
|
Gender, male % |
71 |
|
Height, cm, mean (SD) |
164 (7) |
|
Weight, kg, mean (SD) |
66 (10) |
|
Body mass index, kg/m2, mean (SD) |
24 (4) |
|
Time since stroke, years, mean (SD) |
6 (3) |
|
Ischaemic/haemorrhage, n |
44/58 |
|
Hypertensive medications, % |
75 |
|
Diabetic medications, % |
21 |
|
Brachial systolic blood pressure, mmHg, mean (SD) |
124 (13) |
|
Brachial diastolic blood pressure, mmHg, mean (SD) |
77 (9) |
|
Brachial pulse pressure, mmHg, mean (SD) |
46 (12) |
|
Central systolic blood pressure, mmHg, mean (SD) |
116 (12) |
|
Central diastolic blood pressure, mmHg, mean (SD) |
78 (9) |
|
Central pulse pressure, mmHg, mean (SD) |
38 (12) |
|
Heart rate, beats/min, mean (SD) |
62 (9) |
|
Education levels, years, mean (SD) |
12 (3) |
|
Six-minute walk test, m, mean (SD) |
228 (25) |
|
Muscular strength, kg, mean (SD) |
30 (6) |
|
Flexibility, cm, mean (SD) |
16 (9) |
|
Balance, cm, mean (SD) |
23 (5) |
|
Mini Mental State Examination, p, mean (SD) |
26 (2) |
|
Augmentation index at 75 bpm, %, mean (SD) |
24 (8) |
|
Pulse wave velocity, m/s, mean (SD) |
10 (2) |
|
SD: standard deviation. |
|
|
Table II. Associations among physical fitness, arterial stiffness and cognitive function |
||||||
|
AIx75 |
PWV |
Flexibility |
6MWT |
Muscular strength |
Balance |
|
|
PWV |
0.20* |
|||||
|
Flexibility |
–0.12 |
–0.54** |
||||
|
6MWT |
–0.14 |
–0.55** |
0.35** |
|||
|
Muscular strength |
–0.38** |
–0.46** |
0.35** |
0.55** |
||
|
Balance |
–0.05 |
–0.46** |
0.51** |
0.53** |
0.35** |
|
|
MMSE |
–0.01 |
–0.45** |
0.37** |
0.37** |
0.32** |
0.46** |
|
*p < 0.05; **p < 0.01. 6MWT: 6-min walk test; AIx75: Augmentation index at 75 bpm; PWV: pulse wave velocity; MMSE: Mini Mental State Examination. |
||||||
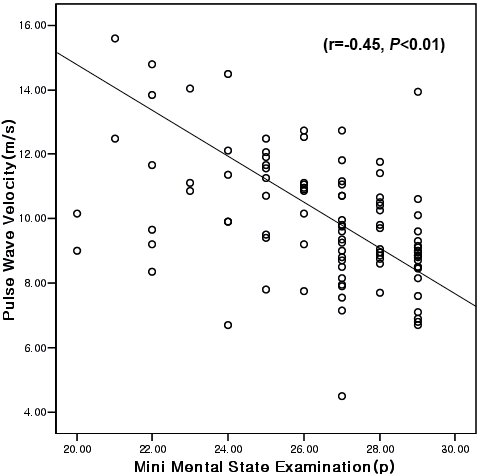
Fig. 2. Correlation between arterial stiffness and cognitive function.
|
Table III. Regression coefficients (95% CI) of cognitive function (MMSE) by arterial stiffness assessed by carotid femoral PWV in chronic stroke patients |
|||
|
Variables |
MMSE |
p-values |
|
|
Β-coefficient |
95% CI |
||
|
Carotid-femoral PWV |
|||
|
Model 1a |
–0.33 |
–0.70 to –0.16 |
0.001 |
|
Model 2b |
–0.11 |
–0.43 to 0.17 |
0.396 |
|
aAdjusted for age, age, BMI, duration of stroke, education, systolic blood pressure, cigarette smoking and medications. bAdjusted for variables in model 1 plus physical fitness. PWV: pulse wave velocity; BMI: body mass index; 95% CI: 95% confidence interval; MMSE: Mini Mental State Examination. |
|||
DISCUSSION
The present study investigated whether the relationship between arterial stiffness and cognitive function in patients with chronic stroke is influenced by physical fitness. The findings indicate that in patients with stroke: (i) physical fitness was positively associated with cognitive function; (ii) physical fitness was inversely associated with arterial stiffness; (iii) arterial stiffness was inversely associated with cognitive function. After adjustment for physical fitness, the association between arterial stiffness and cognitive function was attenuated to non-significance. Thus, of the factors in the casual pathway between arterial stiffness and cognitive function, physical fitness is the one that explained the mechanisms underlying the association between arterial stiffness and cognitive function in patients with stroke. High physical fitness may have a favourable effect on cognitive function in patients with stroke via its favourable effects on vascular function.
In this study, we noted a significant inverse correlation between carotid-femoral PWV and cognitive function. This result supports previous studies that reported a significant inverse correlation between PWV and global cognitive function (29). Increased arterial stiffness is an independent predictor for brain white matter hyperintensities and may contribute to cerebral hypoperfusion, which is related to cognitive impairment (3). Moreover, the results of the regression analysis in this study indicated that the relationship between carotid-femoral PWV and cognitive function was significantly affected by physical fitness factors. This is the first study to suggest that the relationship between arterial stiffness and cognitive function in patients with stroke may be affected by physical fitness.
Physical activity/high physical fitness is associated with improved cognitive function. Physical activity/physical fitness is also known to improve many CVD risk factors associated with both cognitive function and vascular function, such as body weight, blood pressure, inflammation, and insulin resistance (30, 31). Thus, high physical fitness and its favourable effects on vascular function may be associated with improvements in cognitive function (32).
Increased arterial stiffness and cognitive impairment are frequently seen in elderly people and stroke patients (18, 33). With ageing of populations worldwide, increased arterial stiffness and cognitive impairment will be a serious social, health and economic issue in the near future. Over 60% of all cerebrovascular patients have a certain degree of cognitive impairment, with a subsequent 30–50% progressing to develop dementia (34). On the other hand, as indicated from this study, high physical fitness may mediate the association between increased arterial stiffness and decreased cognitive function in patients with chronic stroke. Therefore, maintaining a high level of physical fitness through regular physical activity may help prevent cognitive decline caused by increased arterial stiffness.
Previous studies have reported an inverse association between AIx and cognitive function (35). However, the present study did not support such a relationship, and this is consistent with more recent findings from Mitchell et al. (36) in older adults. AIx is an indicator of central systolic loading and overall global measure of ventricular-vascular coupling. AIx is influenced by numerous factors that may affect the confluence of forward and backward travelling pressure waves (arterial stiffness/impedance, left venticular function/suction, reservoir function, ejection duration, height) (37, 38). The relationship between aortic AIx and cerebral perfusion is complex, as reflected pressure waves in the aorta may enter the carotid and cerebral vasculature as forward pressure waves. Therefore, aortic AIx may not accurately capture carotid-cerebral haemodynamic burden. Further research is needed into the contribution of wave reflections (measured using more refined methods such as wave separation analysis or wave intensity analysis) to cognitive function.
Study limitations
The present study has several limitations. First, subjects were recruited from a specific geographical area, thus the results may not be generalizable to other areas or ethnic groups. Secondly, since this is a cross-sectional study, causality cannot be directly determined, only indirectly inferred. In addition, cardiorespiratory fitness was not measured directly using reference standard methods, such as VO2max with metabolic gas analysis. However, this study is of value, in that it is the first of its kind to suggest that the association between arterial stiffness and cognitive function in patients with stroke may be influenced by physical fitness. Thirdly, since brain images could not be taken, it was not possible to determine the severity of the actual microvascular damage to the brain.
Conclusion
These findings confirm that the association between increased arterial stiffness and decreased cognitive function may be mediated by physical fitness in patients with chronic stroke. Therefore, this study suggests that physical fitness is an important factor affecting the relationship between arterial stiffness and cognitive function.
The authors declare no conflicts of interest.
REFERENCES
