Céline Bonnyaud, MSc1,2, Didier Pradon, PhD1, Julien Boudarham, MSc1, Johanna Robertson, PhD1, Nicolas Vuillerme, PhD2,3 and Nicolas Roche, MD, PhD1
From the 1GRCTH, EA4497, CIC-IT 805, Raymond Poincaré Hospital, Garches, Versailles Saint Quentin en Yvelines University, 2AGIM Laboratory, FRE 3405 CNRS/UJF Grenoble/EPHE, La Tronche, 3Institut Universitaire of France, Paris, France
OBJECTIVE: To evaluate the effects of a 20-min gait training session using the Lokomat® combined with a negative kinematic constraint on the non-paretic limb and a positive kinematic constraint on the paretic limb, on peak knee flexion and other biomechanical parameters in chronic hemiparetic subjects.
DESIGN: Preliminary study, before–after design.
SUBJECTS: Fifteen hemiparetic subjects.
METHODS: Subjects were evaluated using 3-dimensional gait analysis before, immediately after the end of the training, and after a 20-min rest period. The positive constraint increased the range of motion of the paretic limb (hip and knee), while the negative constraint reduced the range of motion of the non-paretic limb (hip and knee).
RESULTS: Peak knee flexion and other, kinematic, kinetic and spatiotemporal, parameters were significantly improved following the training session. These positive effects occurred predominantly in the paretic limb. Moreover, there was no worsening of biomechanical parameters of the non-paretic limb despite the use of negative constraint on this limb. These effects persisted for at least 20 min following the end of the gait training session.
CONCLUSION: This type of training may be effective to improve gait in hemiparetic patients. A larger investigation of the training programme is justified.
Key words: stroke; Lokomat®; gait training; biomechanical gait parameters; motor after-effects.
J Rehabil Med 2014; 46: 00–00
Correspondence address: Bonnyaud Céline, Laboratoire d’analyse du mouvement EA 4497, CIC-IT 805, Hôpital Raymond Poincaré, 104 Bld Raymond Poincaré, FR-92380, Garches, France. E-mail: celine.bonnyaud@ens.uvsq.fr
Accepted Aug 27, 2013; Epub ahead of print Oct 25, 2013
Introduction
The majority of patients with hemiparesis following stroke have residual gait impairments (1–3). Several rehabilitation techniques have been shown to be effective in improving gait function in stroke patients. However, no particular technique has been shown to be more effective than another (4–7). In recent years, many mechanical and electronic systems have been developed to assist movement in hemiparetic patients. Rehabilitation using these systems is based on the concepts of repetitive, intensive, task-orientated training, which has been shown to be effective (8–10).
Among these systems, the Gait Trainer® and the Lokomat® (Hocoma) (which provide mechanical assistance to limb movement with body weight support) have been developed to improve gait in patients with neurological pathologies. The few existing studies have not shown any differences in effectiveness between the use of one of these assistive gait devices and conventional therapy (7, 11); however, Morone et al. (12) suggested that combining robotic therapy with conventional therapy may be more effective than conventional therapy alone. Studies evaluating the effects of gait training using the Lokomat® have shown contradictory results. Schwartz et al. (13) and Hidler et al. (14) showed that use of the Lokomat® did not improve spatiotemporal gait parameters or performance on the 6-min walk test in stroke patients. In contrast, Mayr et al. (15) and Husemann et al. (16), respectively, found an increase in performance on the 6-min walk test and an increase in the duration of paretic-limb stance phase as a percentage of the gait cycle following a training session using the Lokomat®. Westlake et al. (17) found similar effects when they compared Lokomat® training with treadmill training with body-weight support in chronic hemiparetic patients.
Thus, there are currently no conclusive results regarding the effectiveness of Lokomat® training compared with conventional training on spatiotemporal gait parameters. The differences in results between the different studies could be explained by the difference in degree of chronicity of the patients as well as by the settings used during the Lokomat® gait training session (range of motion, level of guidance, speed), or they may indicate a lack of consistent benefit of Lokomat® therapy.
By means of its exoskeleton design, the Lokomat® may have a particular effect on the gait pattern. As far as we are aware, no studies have assessed its effects on kinematic and kinetic gait parameters in hemiparetic patients.
The Lokomat® programmes are designed to assist and facilitate symmetrical gait-like movements, and thus to create a normal gait pattern. However, it is possible that facilitation of a different type of gait pattern may be more effective. Indeed, constraint-induced movement therapy (CIMT), based on the constraint of a non-affected part of the body such as the upper limb (18,19), lower limb (20, 21) or trunk (22) to force use of the affected part, has been shown to be an effective rehabilitation technique for the upper limb (18, 19, 22). In contrast, the effect of such an approach on gait has rarely been studied (20, 21). Marklund et al. (20) found an improvement in motor function, functional gait tests and weight-bearing symmetry in hemiparetic subjects after a gait training programme, which involved wearing an orthosis on the non-paretic lower limb to limit knee flexion and extension. More recently, Regnaux et al. (21) found improvements in kinematic, kinetic and spatio-temporal parameters after a 20-min gait training session on a treadmill with a mass applied to the non-paretic ankle in hemiparetic subjects. In both studies, the improvements in gait parameters occurred immediately after the end of the training session and were maintained or further increased 20 min later. These innovative studies suggest that the use of negative constraint on the non-paretic lower limb of hemiparetic patients during a gait training session could be a useful rehabilitation technique for the improvement in gait.
The design of the Lokomat® robotic system allows both positive constraint (i.e. an increase in range of motion to facilitate movement) and negative constraint (i.e. a reduction in range of motion to restrain the movement) to be applied. Based on data in the literature, it would appear that a gait training paradigm, which involves imposing positive constraint on the paretic lower limb and negative constraint on the non-paretic lower limb, may be effective for rehabilitation. Such a paradigm has, until now, never been studied. The aim of this study was therefore to evaluate the effects of a 20-min gait training session using the Lokomat® set so as to provide negative constraint on the non-paretic limb and positive constraint on the paretic limb on biomechanical gait parameters (kinematic, kinetic and spatiotemporal parameters) in stroke patients. Since we imposed a large range of motion on the paretic limb, we hypothesized that the principal effect of this training paradigm would be on kinematic gait parameters. Because knee flexion during the swing phase is frequently limited in hemiparetic patients and is often the target of treatment techniques, we chose this parameter as the primary outcome measure for the study. In addition, we hypothesized that this Lokomat® training paradigm would also improve other kinematic, kinetic and spatiotemporal gait parameters on the paretic side.
MethodS
Subjects
Fifteen hemiparetic subjects were included (9 with right hemiparesis, 6 with left hemiparesis; 11 males, 4 females, mean age 53.4 years (standard deviation (SD) 13.3). Patients were recruited from the department if they were hospitalized and from medical outpatient consultations in our hospital. The inclusion criteria were: a cerebral vascular lesion affecting a single hemisphere which occurred more than 6 months previously; able to walk 10 m with no assistance or assistive device and for 20 min non-stop. All patients who fulfilled the inclusion criteria between December 2011 and April 2012 were asked to participate and all agreed. All subjects gave informed consent before inclusion. The study was carried out according to “the ethical codes of the World Medical Association” (Declaration of Helsinki) and was approved by the local ethics committee (CPP Ile de France – Ambroise Paré).
Experimental procedure
Each subject underwent 20 min of gait training with the Lokomat® (wearing their own shoes), during which a negative kinematic constraint was applied to the non-paretic limb (the range of motion of the hip and knee joints was reduced) and a positive kinematic constraint was applied to the paretic limb (the range of motion of the hip and knee joints was increased). The training sessions were carried out using the basic version of the Lokomat®. Gait speed was set to 1.5 km/h for each patient. The Lokomat® was set so as to impose the largest possible range of flexion/extension on the paretic hip and knee and the smallest range of flexion/extension on the non-paretic hip and knee. When the application of the 2 constraints was too difficult for the patient to follow the movement, the security system stopped the device. Constraint settings were thus individualized for each patient with the aim being to create the largest degree of hip and knee asymmetry between the paretic and non-paretic limbs without activating the security system. The settings for each patient are listed in Table I. Guidance was set to 100% for each patient in order to ensure that the full range of imposed asymmetry was carried out. The patient was instructed to participate as actively as possible, since Peurala et al. (23) demonstrated the importance of therapist instructions during gait training in order to optimize the effects of rehabilitation. All the gait training sessions were supervised by the same physiotherapist.
|
Table I. Percentage of asymmetry induced by the Lokomat® |
||
|
Subject |
Hip asymmetry Mean (SD) |
Knee asymmetry Mean (SD) |
|
1 |
32.6 (3.4) |
34.2 (3.1) |
|
2 |
27.3 (3.5) |
31.6 (5.5) |
|
3 |
20.7 (0.0) |
40.1 (3.9) |
|
4 |
30.7 (8.1) |
61.4 (15.9) |
|
5 |
29.3 (7.2) |
34.1 (9.3) |
|
6 |
40.4 (6.2) |
46.4 (8.7) |
|
7 |
35.3 (4.4) |
33.5 (10.4) |
|
8 |
25.7 (4.3) |
28.2 (3.4) |
|
9 |
46.5 (15.6) |
54.2 (26.6) |
|
10 |
38.7 (8.9) |
45.0 (12.3) |
|
11 |
47.3 (5.9) |
36.4 (13.7) |
|
12 |
18.7 (6.2) |
45.9 (8.5) |
|
13 |
31.9 (14.9) |
35.6 (17.4) |
|
14 |
15.7 (3.7) |
21.2 (4.9) |
|
15 |
50.8 (5.8) |
62.1 (16.3) |
|
A value of zero indicates perfect symmetry. Higher values indicate a higher level of asymmetry on the paretic side relative to the non-paretic side. Percentage of asymmetry induced by the Lokomat® is calculated with the symmetry index (SI) defined by Robinson et al. (34), as follows: SI = (ABS(Vparetic – Vnon-paretic)/ABS (0.5 (Vparetic + Vnon-paretic))) × 100 SI: symmetry index as a percentage; Vparetic: biomechanical gait parameter of the paretic lower limb; Vnon-paretic: biomechanical gait parameter of the non-paretic lower limb; SD: standard deviation; ABS: absolute value. |
||
Assessments
Clinical. Before the training session, the patients underwent a clinical evaluation of their level of independence in activities of daily living (Barthel Index) and their gait ability (New Functional Ambulation Classification NFAC) (24, 25).
Instrumented. Each patient underwent 3 quantified 3-dimensional gait analyses: before training (Baseline), immediately after training (Post 0) and after a 20 min rest (Post 20). The gait analyses were carried out using a motion analysis system consisting of 8 optoelectronic cameras (Motion Analysis Corporation, Santa Rosa, CA, USA, Sampling Frequency 100 Hz) with 2 force platforms (AMTI, Watertown, MA, USA, sampling frequency 1000 Hz). Thirty reflective markers were positioned according to the Helen Hays protocol (26). Subjects walked at their spontaneous velocity wearing their own shoes, in order to maintain the same conditions as during the training in the Lokomat®. Ten gait trials were recorded and averaged for each subject. The signal was filtered at 6 Hz using a low-pass Butterworth filter (27). Orthotrak 6.2.8 (Motion Analysis Corporation, CA, USA) was used to calculate the kinematic and kinetic gait parameters for each gait cycle. The training was carried out in a different room from the gait analyses and patients were pushed in a wheelchair between the rooms. The position of the markers was marked on the skin in order to avoid errors relating to their repositioning. All gait analyses were performed by the same investigator.
Three types of biomechanical gait parameters were studied:
• Kinematic: peak hip and knee flexion and extension and ankle dorsifexion and plantarflexion were calculated for both limbs;
• Kinetic: peak propulsion and braking forces in the sagittal plane and peak vertical ground reaction force (GRF) during stance phase were calculated for both limbs;
• Spatiotemporal: gait velocity, step length, cadence and the percentage of the gait cycle spent in stance and swing phase.
Statistical analysis
A repeated measures analysis of variance (ANOVA) test was used. Time (at Baseline, Post 0 and Post 20) were analysed for each variable. p < 0.05 was considered significant in each case. A post hoc analysis using Fisher’s least significant difference was carried out on the significant comparisons. To check the robustness of the results, Friedman’s test had also been applied, followed by Wilcoxon signed-rank test as a post hoc test. Statistical analysis was carried out using Statistica 7.1 software.
Results
Kinematic gait parameters
The kinematic gait parameters for both limbs are shown in Table II.
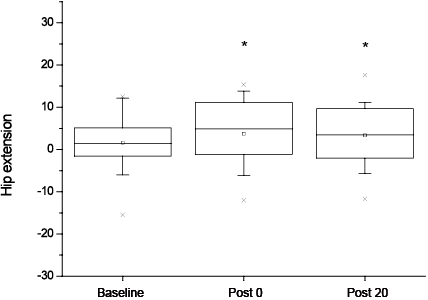
The primary outcome measure: the mean standard deviation (SD) peak knee flexion during swing phase was significantly increased following the training session (41.5º (SD 8.4) at Baseline, 44.1º (SD 7.5) at Post 0 (p = 0.01) and 43.9º (SD 7.6) at Post 20 (p = 0.02)). In addition, the repeated measures ANOVA showed a significant effect of time on several other kinematic parameters; there was a significant increase in peak hip extension (–1.6º (SD 7.4) at Baseline, –3.7º (SD 7.8) at Post 0 (p = 0.01) and –3.4º (SD 7.6) at Post 20 (p = 0.04)), and a decrease in the mean peak knee extension (2.9º (SD 8.9) at Baseline, 1.1º (SD 9.9) at Post 0 (p = 0.03) and 0.8º (SD 9.9)at Post 20 (p = 0.008)), in the paretic limb. For each parameter, the post hoc analysis showed a difference between Baseline and Post 0 and Baseline and Post 20, but not between Post 0 and Post 20. The improvements in peak knee flexion and peak hip extension in the paretic limb are shown in Figs 1 and 2, respectively. Figures are shown as boxes, with minimum, maximum, mean and 1% and 99% of the values. It can be noted that peak hip flexion of the paretic lower limb was not modified by the gait training session.
|
Table II. Kinematic gait parameters |
|||||
|
Baseline Mean (SD) |
Post 0 Mean (SD) |
Post 20 Mean (SD) |
p-values |
Difference Post 20 – Baseline Mean (95% CI) |
|
|
Peak hip flexion, paretic side, º |
31.5 (6.6) |
31.3 (5.3) |
31.5 (5.7) |
0.91 |
–0.02 (–1.31 to 1.28) |
|
Peak hip extension, paretic side, º |
–1.6 (7.4) |
–3.7 (7.8)* |
–3.4 (7.6)* |
0.03 |
–1.74 (–3.77 to 0.28) |
|
Peak knee flexion, paretic side, º |
41.5 (8.4) |
44.1 (7.5)* |
43.9 (7.6)* |
0.02 |
2.47 (–0.04 to 4.99) |
|
Peak knee extension, paretic side, º |
2.9 (8.9) |
1.1 (9.9)* |
0.8 (9.9)* |
0.02 |
–2.13 (–3.98 to –0.28) |
|
Peak ankle dorsiflexion, paretic side, º |
16.9 (5.9) |
17.2 (3.8) |
17.2 (4.0) |
0.88 |
0.26 (–1.34 to 1.86) |
|
Peak ankle plantarflexion, paretic side, º |
–8.6 (7.8) |
–9.0 (7.3) |
–9.8 (7.1) |
0.06 |
–1.24 (–2.33 to –0.15) |
|
Peak hip flexion, non-paretic side, º |
39.8 (5.7) |
40.0 (5.3) |
40.9 (5.3) |
0.19 |
1.1 (–0.38 to 2.62) |
|
Peak hip extension, non-paretic side, º |
–8.7 (6.4) |
–10.2 (5.9) |
–9.9 (6.9) |
0.27 |
–1.28 (–3.73 to 1.16) |
|
Peak knee flexion, non-paretic side, º |
66.7 (4.5) |
67.3 (3.6) |
67.6 (3.4) |
0.08 |
0.89 (–0.04 to 1.83) |
|
Peak knee extension, non-paretic side, º |
4.8 (4.5) |
4.9 (4.5) |
4.4 (4.6) |
0.44 |
–0.40 (–1.44 to 0.63) |
|
Peak ankle dorsiflexion, non-paretic side, º |
21.6 (3.1) |
21.8 (2.7) |
21.8 (2.5) |
0.73 |
0.18 (–0.44 to 0.80) |
|
Peak ankle plantarflexion, non-paretic side, º |
–8.7 (4.9) |
–8.8 (5.1) |
–9.2 (4.9) |
0.67 |
–0.48 (–1.63 to 0.66) |
|
p-values correspond to analysis of variance (ANOVA) analysis. *Significant difference (p < 0.05) between baseline and Post 0 and between baseline and Post 20. No difference between Post 0 and Post 20. Baseline: before the training; Post 0: immediately after the training; Post 20: after 20 min rest; 95% CI: 95% confidence intervals; SD: standard deviation. |
|||||
Fig. 1. Knee flexion on the paretic side at Baseline, Post 0 and Post 20 shown with min–max (┬┴ ), mean () and 1% and 99% (×) of the values (*significant difference at p < 0.05 between Baseline and Post 0 and between Baseline and Post 20). Baseline: before training; P0: immediately after training; P20: after 20 min of rest.
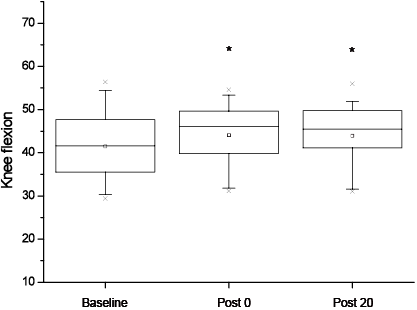
Fig. 2. Hip extension on the paretic side at Baseline, Post 0 and Post 20 shown with min–max (┬┴ ), mean () and 1% and 99% (×) of the values (*significant difference at p < 0.05 between Baseline and Post 0 and between Baseline and Post 20). Baseline: before training; P0: immediately after training; P20: after 20 min of rest.
There were no statistically significant differences for any of the kinematic parameters analysed for the non-paretic limb.
Friedman test confirmed the findings for peak knee flexion, the primary outcome measure (p = 0.02; Wilcoxon signed-rank test showed a significant difference between Baseline and Post 20), and for peak hip extension (p = 0.03; Wilcoxon signed-rank test showed a significant difference between Baseline and Post 0) and peak knee extension (p = 0.02; Wilcoxon signed-rank test showed a significant difference between Baseline and Post 20) on the paretic side. A significant difference was also found for peak ankle plantarflexion on the paretic side (p = 0.03) with Friedman test (Wilcoxon signed-rank test confirmed this difference between Baseline and Post 20).
Kinetic gait parameters
The results of all the kinetic gait parameters studied for both limbs are shown in Table III. Following training, the repeated measures ANOVA showed a significant effect of time on several kinetic parameters. Indeed, there was a significant increase in peak propulsion force for both limbs (paretic limb: 0.058 N/kg at Baseline, 0.064 N/kg at Post 0, and 0.068 N/kg at Post 20 (p = 0.006); non-paretic limb: 0.156 N/kg at Baseline, 0.169 N/kg at Post 0 (p = 0.03), and 0.169 N/kg at Post 20 (p = 0.03)). There was also a significant increase in peak braking force in both limbs (paretic limb: –0.145 N/kg at Baseline, –0.174 N/kg at Post 0 (p = 0.007) and –0.157 N/kg at Post 20; non-paretic limb: –0.118 N/kg at Baseline, –0.127 N/kg at Post 0 and –0.134 N/kg at Post 20 (p = 0.007)). Post hoc analysis showed differences between Baseline and Post 20 for peak propulsion force of the paretic limb and between Baseline and Post 0 and Post 20 for peak propulsion force of the non-paretic limb. It also showed a significant difference between Baseline and Post 0 for peak braking force on the paretic side and between Baseline and Post 20 for the non-paretic limb. There were no significant differences in peak vertical GRF during stance phase in either limb.
|
Table III. Kinetic gait parameters |
|||||
|
Baseline Mean (SD) |
Post 0 Mean (SD) |
Post 20 Mean (SD) |
p-values |
Difference Post 20 – Baseline Mean (95% CI) |
|
|
Vertical GRF: Total support phase, paretic side, N/kg |
0.782 (0.05) |
0.789 (0.03) |
0.791 (0.03) |
0.06 |
0.01 (–0.01 to 0.02) |
|
Peak propulsion: Final support phase, paretic side, N/kg |
0.058 (0.02) |
0.064 (0.03) |
0.068 (0.03)* |
0.02 |
0.01 (0.00 to 0.02) |
|
Peak braking: Initial support phase, paretic side, N/kg |
–0.145 (0.05) |
–0.174 (0.06)* |
–0.157 (0.06) |
0.02 |
–0.01 (–0.03 to 0.01) |
|
Vertical GRF: Total support phase, non-paretic side, N/kg |
0.830 (0.03) |
0.829 (0.03) |
0.837 (0.03) |
0.15 |
0.01 (–0.02 to 0.02) |
|
Peak propulsion: Final support phase, non-paretic side, N/kg |
0.156 (0.06) |
0.169 (0.04)* |
0.169 (0.05)* |
0.04 |
0.02 (0.01 to 0.03) |
|
Peak braking: Initial support phase, non-paretic side, N/kg |
–0.118 (0.05) |
–0.127 (0.05) |
–0.134 (0.05)* |
0.02 |
–0.02 (–0.03 to –0.01) |
|
p-values correspond to analysis of variance (ANOVA) analysis. *Significant difference (p < 0.05) between baseline and Post 0 and between baseline and Post 20. No difference between Post 0 and Post 20. Baseline: before the training; Post 0: immediately after the training; Post 20: after 20 min rest; 95% CI: 95% confidence intervals; SD: standard deviation. |
|||||
Friedman test confirmed the findings obtained with ANOVA for the peak propulsion force (p = 0.01; Wilcoxon signed-rank test showed a significant difference between Baseline and Post 20) and the peak braking force (p = 0.008; Wilcoxon signed-rank test showed also a significant difference between Baseline and Post 0) on the paretic side, but did not highlight a significant difference for these parameters on the non-paretic side.
Spatiotemporal gait parameters
The spatiotemporal gait parameters for each limb are shown in Table IV. Following the training session, the ANOVA showed a significant effect of time on gait velocity, cadence and paretic step length. There was a significant increase in gait velocity (76.4 cm/s (SD 21.3) at Baseline, 81.3 cm/s (SD 17.9) at Post 0 (p = 0.02) and 81.9 cm/s (SD 19.0) at Post 20 (p = 0.01)), cadence (86.4 steps/min (SD 13.5) at Baseline, 89.6 steps/min (SD 11.9) at Post 0 (p = 0.02) and 89.6 steps/min (SD 12.2) at Post 20 (p = 0.02)) and paretic step length (52.1 cm (SD 9.3) at Baseline, 55.4 cm (SD 6.9) at Post 0 (p = 0.04), 55.7 cm (SD 6.9) at Post 20 (p = 0.03)). For each of these parameters, post hoc analysis showed differences between Baseline and Post 0 and Baseline and Post 20, but not between Post 0 and Post 20.
|
Table IV. Spatiotemporal gait parameters |
|||||
|
Baseline Mean (SD) |
Post 0 Mean (SD) |
Post 20 Mean (SD) |
p-values |
Difference Post 20 – Baseline Mean (95% CI) |
|
|
Speed, cm |
76.4 (21.3) |
81.3 (17.9)* |
81.9 (19.0)* |
0.02 |
5.4 (1.4; 9.5) |
|
Cadence, step/min |
86.4 (13.5) |
89.6 (11.9)* |
89.6 (12.2)* |
0.04 |
3.1 (0.4; 5.9) |
|
Step length, paretic side, cm |
52.1 (9.3) |
55.4 (6.9)* |
55.7 (6.9)* |
0.04 |
3.6 (0.6; 6.5) |
|
Percentage support phase, paretic side, % |
60 (4.0) |
59.3 (3.3) |
59.1 (3.3) |
0.12 |
–0.9 (–1.9; 0.2) |
|
Percentage swing phase, paretic side, % |
39.9 (4.0) |
40.7 (3.3) |
40.9 (3.3) |
0.12 |
0.9 (–0.2; 1.9) |
|
Step length, non-paretic side, cm |
47.7 (8.0) |
50.6 (5.0) |
51.4 (4.4) |
0.06 |
3.7 (0.5; 6.9) |
|
Percentage support phase, non-paretic side, % |
69.1 (4.4) |
68.1 (3.1) |
68.1 (3.0) |
0.12 |
–0.9 (–2.1; 0.1) |
|
Percentage swing phase, non-paretic side, % |
30.9 (4.4) |
31.9 (3.2) |
31.9 (3.0) |
0.12 |
0.9 (–0.2; 2.1) |
|
p-values correspond to analysis of variance (ANOVA) analysis. *Significant difference (p < 0.05) between baseline and Post 0 and between baseline and Post 20. No difference between Post 0 and Post 20. Baseline: before the training; Post 0: immediately after the training; Post 20: after 20 min rest; 95% CI: 95% confidence intervals; SD: standard deviation. |
|||||
There was no significant change in the percentage of the gait cycle spent in stance or swing phase on the paretic limb. There was no statistically significant difference for any of the spatiotemporal parameters analysed for the non-paretic limb.
Friedman test did not find any significant difference for all the spatio-temporal gait parameters assessed.
Clinical assessment
The median Barthel index score was 95. The median score of the modified Functional Ambulation Classification was 7.
Discussion
The main aim of this study was to evaluate the effect of a Lokomat® constraint training paradigm on peak knee flexion in chronic hemiparetic subjects. The secondary aims were to evaluate the after-effects of the single training session on other biomechanical gait parameters (kinematic, kinetic and spatiotemporal). To our knowledge, this is the first study to use such a rehabilitation paradigm. It is also the first study to evaluate the immediate (Post 0) and short-term after-effects (Post 20) induced by a gait training session using the Lokomat® on biomechanical gait parameters. The results showed that a single Lokomat® gait training session significantly improved peak knee flexion during the swing phase of the gait cycle, as well as other kinematic, kinetic and spatiotemporal gait parameters. In addition, the results of this study showed that a negative kinematic constraint applied to the non-paretic lower limb did not alter its biomechanical parameters.
Effects on the paretic limb
Peak knee flexion during the swing phase increased significantly on the paretic side following a Lokomat® gait training session using a constraint paradigm in stroke patients. This suggests that negative kinematic constraint on the non-paretic limb, coupled with positive constraint on the paretic limb, increased knee flexion towards a more normal range of motion. As this paradigm also increased peak propulsion of the paretic lower limb, but did not modify peak hip flexion, it might be hypothesized that the increased knee flexion resulted from an improvement in knee angular velocity at toe-off. Although ANOVA statistical analysis did not find a significant increase of peak ankle plantarflexion on the paretic side, Friedman test showed a significant improvement in this parameter after the training session. As ankle plantarflexion could be associated with increase peak propulsion force, it might be hypothesized that these 2 mechanisms, which are concomitant during the final support push-off phase (28), contributed to increase peak knee flexion in the swing phase after the training session. Peak hip extension was also increased following the training session. The improvement in this kinematic parameter was measured with the 2 different statistical analyses performed; thus we have confidence in this result, which is in accordance with the results of Hidler et al.’s study (29). These authors compared kinematic changes in 6 healthy subjects following a gait training session on the Lokomat® and on a treadmill and found similar findings. They showed that the kinematic changes that occurred following the Lokomat® training were similar to those following treadmill training, but that there was a significantly greater increase in peak hip extension and range of hip and ankle flexion-extension following the Lokomat® training.
The results of this study highlighted that both peak hip extension and peak knee flexion are increased after a gait training session performed on the Lokomat® with a specific paradigm. These kinematic improvements are also associated with an improvement in gait speed (which is significant on ANOVA statistical analysis, but not with the Friedman test). This difference could be explained by the nature of the statistical analysis performed; i.e. a parametric test on the one hand and a non-parametric test on the other hand. Independently of the statistical analysis performed, the gait speed increased after the gait training session on the Lokomat®. This result is in accordance with those of Westlake & Patten (17), who found a significant speed improvement following a Lokomat® training in stroke patients. It can also be noticed that when stroke subjects are asked to walk faster, they increase both hip flexion/extension angles and knee flexion angle (30, 31). Taken together, these results suggest that these 2 kinematic parameters (hip flexion/extension range of motion and peak knee flexion in the swing phase) might be the kinematic parameters that are: (i) the most sensitive to change, and (ii) the best “controlled” by patients.
Effects on the non-paretic side
The application of negative constraint on the non-paretic limb could theoretically have worsened biomechanical gait parameters of the non-paretic limb. However, our results did not confirm this hypothesis. Indeed, there was no worsening of any of the parameters evaluated. Friedman analysis showed no significant change on the non-paretic side, and ANOVA analysis showed significant improvement on the non-paretic side following the Lokomat® gait training. The modifications of kinetic values observed with ANOVA analysis, showing that propulsion and braking force are increased on the non-paretic side, suggest compensation between both lower limbs. Indeed during gait, when one lower limb is in the initial double stance phase, the other limb is in the final double stance phase and vice versa. In order to maintain balance, it appears important that any increase in propulsion force on one side is compensated for by an increased braking force on the other side (32). As such, it is possible that an increase in propulsion force on the paretic limb during the final double stance phase is compensated for by an increase in braking force on the non-paretic limb, and vice versa. These results suggest strongly that a gait training session performed on Lokomat®, combined with a negative constraint, does not alter the kinetic parameters of the limb to which it is applied.
Duration of the effects
Wilcoxon signed-rank post hoc test particularly highlighted improvements in short-term kinematics on the paretic side (significant difference between Baseline and Post 20). Fisher’s less significant difference post hoc test showed improvements in immediate and short-term kinematics (significant difference between Baseline and Post 0 and between Baseline and Post 20). Taken together, these 2 statistical methods support the results, indicating that the gait training session performed with this particular paradigm significantly improved kinematic and kinetic gait parameters 20 min after the end of the training. This is in agreement with a study by Kim et al. (33), who found that gait training using a robotic exoskeleton in healthy subjects led to kinematic after-effects that lasted for at least 2 h following the training. Regnaux et al. (21) also found that 20 min of treadmill training with a constraint applied on the non-paretic limb led to significant immediate improvements in kinematic and kinetic parameters of the paretic limb in 10 hemiparetic patients, which persisted, or even continued to increase 20 min later. Further studies are necessary to determine the exact duration of the after-effects, as well as to evaluate whether a series of gait training sessions with the Lokomat®, coupled with positive constraint on the paretic lower limb and negative constraint on the non-paretic limb, could induce long-term effects on gait.
Limitations
This pilot study was designed to evaluate an original paradigm of kinematic constraint to improve gait pattern in hemiparetic patients. We have confidence in the kinematic and kinetic improvements on the paretic side following the training, since both statistical analyses found same significant results. Further studies with more subjects are necessary to draw conclusions about spatio-temporal gait parameters and to determine whether this type of training is more effective than conventional training. Further studies are also necessary to assess the effects of several training sessions using this constraint paradigm, since the confidence intervals for improvements with a single session performed in 15 subjects were quite wide. The patients included in this study had a good level of recovery, as shown by the NFAC score and gait velocity at baseline (76.4 cm/s). They were all capable of walking at least 20 min without stopping. The results are therefore applicable only to patients with a moderate to good motor recovery. It could be argued that the results found during the training session were related to the velocity imposed on the subject. This hypothesis, however, is unlikely to be true because of the fact that the mean baseline gait velocity was 2.5 km/h, while the training was carried out at 1.5 km/h.
The Lokomat® was designed to assist gait and to impose a symmetrical pattern close to normal. Although it is possible to set the Lokomat® to create an asymmetrical pattern, beyond a certain level of asymmetry the system prevents this, and the training is stopped. Therefore, in this study, each patient underwent the training with the largest possible asymmetry they could cope with. This varied between patients (mean30% asymmetry at the hip and 42% at the knee). This does not invalidate the results of the study, particularly since the patients with the lowest percentage of asymmetry either at the hip or the knee had similar changes following the training to those who carried out the training with larger levels of asymmetry.
The basic version of the Lokomat® does not quantify the degree of participation of the patient in the training, which is why a level of guidance of 100% was used in order to ensure that the patients actually went through the whole range of motion set by the kinematic constraints. Verbal encouragement was given to all patients during the training session in order to promote active participation. However, it is possible that the patients did not all participate to the same degree, which could mask certain effects. It can also be argued that the after-effects induced by the gait training session performed on the Lokomat® could be either due to the paradigm used or due to the gait training on the Lokomat® itself. The results of Hidler et al. (29) showed that after-effects induced by a gait training session performed on the Lokomat® induced changes in hip range of motion, but did not find any modifications in peak knee flexion of the paretic lower limb (which was the case in the present study). This last result suggests that the biomechanical after-effects observed are probably due to the paradigm used.
Conclusion
The results of this study suggest that a Lokomat® constraint gait training session, improves peak knee flexion of the paretic limb as well as gait velocity, paretic-limb step length, peak hip extension on the paretic side, and peak propulsion and braking forces in both limbs. The effects were predominant in the paretic limb and there was no worsening of biomechanical parameters of the non-paretic limb despite the application of negative constraint to this limb. These effects persisted after a rest period of 20 min after the end of the training session. This type of training could constitute a novel therapeutic approach to improving gait velocity as well as gait pattern in hemiparetic patients with moderate to good recovery.
Acknowledgements
The authors would like to thank the patients who participated in this study, and Raphael Zory for his constructive comments.
A grant was awarded for this project by the Coloplast Foundation.
References
