Mirja Vuorenmaa, PT, MSc1, Jari Ylinen, MD, PhD1, Kirsi Piitulainen, PT, MSc1, Petri Salo, PT, PhD1, Hannu Kautiainen, BA2,3, Maija Pesola, MD, PhD4 and Arja Häkkinen, PhD1,5
From the 1Department of Physical Medicine and Rehabilitation, Central Finland Health Care District, Jyväskylä, 2Department of General Practice, Unit of Primary Health Care, Helsinki University Central Hospital, Helsinki, 3Unit of Primary Health Care, Turku University Hospital, Turku, 4Department of Orthopaedics and Traumatology, Central Finland Health Care District and 5Department of Health Sciences, University of Jyväskylä, Jyväskylä, Finland
OBJECTIVE: To evaluate the efficacy of a delayed home exercise programme compared with normal care after primary total knee arthroplasty.
DESIGN: Single-blind, prospective, randomized, controlled trial.
PARTICIPANTS: A total of 108 participants (61% females, mean age 69 years [standard deviation 8.7]), were randomized to a home-based exercise group (EG, n = 53) or to a control group (CG, n = 55).
METHODS: Two months post-operatively, the EG received a home exercise programme, while the CG received no additional guidance. The outcome measurements were: pain and disability, measured using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC); health-related quality of life (HRQoL), measured using the Short Form-36 questionnaire (SF-36); maximal walking speed; isometric knee muscle strength; and the Timed Up and Go (TUG) test. Measurements were made at baseline and at 12 months thereafter.
RESULTS: At the 12-month follow-up, maximal walking speed (p < 0.001) and knee flexion strength (p = 0.009) were significantly greater in the EG. Both groups showed similar improvements in all of the WOMAC subscale scores, the SF-36 summary scores and the TUG time.
CONCLUSION: Home-based training was not superior to normal care with regard to pain, disability or HRQoL, but resulted in greater improvement in objectively measured physical performance.
Key words: osteoarthritis; knee replacement; exercise; disability; health-related quality of life; rehabilitation; home programme.
J Rehabil Med 2013; 45: 00–00
Correspondence address: Jari Ylinen, Department of Physical Medicine and Rehabilitation, Central Finland Health Care District, Keskussairaalantie 19, FI-40620 Jyväskylä, Finland. E-mail: jari.ylinen@ksshp.fi
Accepted Aug 20, 2013; Epub ahead of print Nov 12, 2013
INTRODUCTION
The number of joint replacement procedures worldwide has increased considerably in recent years (1, 2). In Finland, there has been a 69% increase in total knee arthroplasty (TKA) during the last decade, with an incidence of primary TKA of more than 180 per 100,000 inhabitants in 2010 (3). The most common reason for TKA surgery is osteoarthritis (OA). TKA is an effective treatment to reduce pain and subjective disability in patients with OA of the knee, but several studies have shown that muscle strength deficiency (4–6) and functional limitations can persist for several years after surgery (7, 8).
A meta-analysis showed that functional physiotherapy exercises after TKA could have small to moderate short-term benefits 3–4 months postoperatively for physical function, range of joint motion and quality of life, but these benefits were no longer evident at 1-year of follow-up (9). Only one of these studies has explored the efficacy of a home-based rehabilitation (10). That study reported encouraging findings that home-based rehabilitation decreased pain and disability equally compared with in-patient rehabilitation. Since that meta-analysis, several new intervention studies have been published, but none of them have studied the effect of unsupervised home-based exercise (11–16).
Home-based exercise programmes have some advantages compared with clinically based supervised exercise interventions, which are expensive, as they require special facilities, equipment and trained personnel (11). Moreover, not all patients are willing to participate in group exercises, due to scheduling and transportation problems.
The aim of this study was to investigate the efficacy of a 12-month home exercise programme on pain, disability (Western Ontario and McMaster Universities Osteoarthritis Index, WOMAC), health-related quality of life (HRQoL) and physical performance, compared with normal care after primary unilateral TKA. We hypothesized that the long-term postoperative home exercise programme would decrease pain and disability and improve HRQoL more than normal care when assessed 14 months after surgery.
PARTICIPANTS AND METHODS
This study was implemented from January 2008 to February 2010. Subjects were recruited from participants selected for TKA in the Central Finland Health Care District, with a population of 270,000, during preoperative orientation visits to the Jyväskylä Central Hospital Out-Patient Clinic. Inclusion criteria were: (i) diagnosed knee OA; (ii) primary arthroplasty of the knee in question; and (iii) age over 18 years. Exclusion criterion were: (i) other surgery for the lower limbs planned to be performed within 12 months; (ii) dementia; (iii) fibromyalgia; (iv) other serious co-morbidities preventing active training; and (v) difficulty visiting a physiotherapist due to a long travelling distance. Of the total of 301 participants screened, 191 were excluded. The most common reason for exclusion was a long travelling distance to follow-up visits, and the most common medical reason was serious heart disease preventing active training. Two participants withdrew from the study before randomization, one due to back surgery planned within 12 months and one due to a frail post-operative condition. A total of 108 volunteer participants were randomized into the exercise group (EG, n = 53) or control group (CG, n = 55), using a randomization list computer-generated by Medstat (17). Block randomization (size of 4) was undertaken separately for men and women by a person not working with the participants. The randomization was performed before baseline measurements.
The questionnaires and physical performance tests were undertaken 2 months after the operations, at the time when the intervention started, and 12 months thereafter, i.e. 14 months after surgery. In addition the SF-36 and WOMAC questionnaires were completed at 6 months after baseline. The intervention profile is shown in Fig. 1.
Fig. 1. Knee arthroplasty study flowchart.
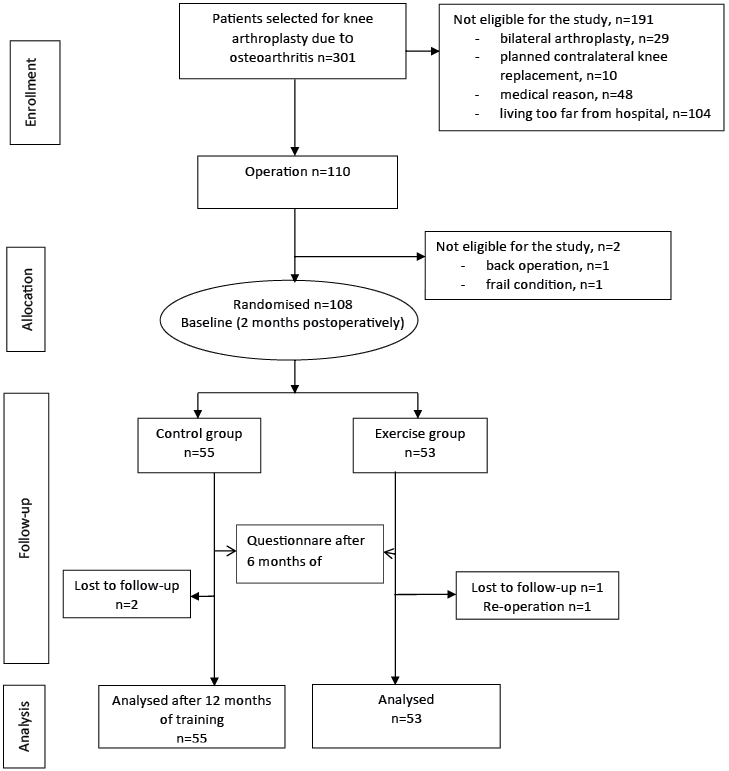
All of the outcome measurements were obtained by two physiotherapists who were blinded to the treatment group assignment. The participants also completed a questionnaire before the operations, concerning their demographic and clinical characteristics.
The study plan was approved by the ethics committee of the Central Finland Care Health District. The study protocol was explained to the participants, who provided written consent prior to participation. The study has been registered in the ClinicalTrials.gov database (NCT00605124).
Operation and early rehabilitation during the first 2 months postoperatively
All of the participants underwent cemented TKA. All of the operations were performed with a tourniquet, which was released after cementing the components, and haemostasis was achieved before closing. Standard medial parapatellar exposure was used. The anterior cruciate ligament, menisci and osteophytes, when present, were removed. The femoral and tibial bone cuts were performed with the help of appropriate jigs, according to preoperative planning. Fitting with a trial prosthesis was performed, and necessary balancing of the ligaments was undertaken. If it was necessary to balance the posterior cruciate ligament, it was scarified, and posterior stabilising components were used. Patellar resurfacing was performed only if the patellar articulating surface was poor, causing patellar maltracking. Postoperative drainage was not routinely performed. Correct positioning of the components was ascertained postoperatively with routine X-rays.
On the second day after the operations, all of the participants were allowed to perform full weight-bearing on the operated leg or as much as they could tolerate. For safety reasons, crutches were recommended for 4–5 weeks after the operations. In accordance with normal procedures, the postoperative hospital stay was one week. On discharge from hospital the participants received advice concerning the application of cold packs and a written exercise programme, which included active and passive knee range-of-motion exercises, knee flexor and extensor exercises, and hip abductor and extensor exercises in the standing position, using the weight of an extremity as resistance. Participants were instructed to perform these exercises 1–2 times per day, with 10–15 repetitions. The participants were also advised to be active, gradually increasing their walking distance over time.
Intervention
The participants randomized to the EG were given individual guidance at baseline (2 months post-operatively) and at 1 and 4 months thereafter by the same physiotherapist. At each visit, they received written information on the exercises and were instructed to keep a weekly exercise diary, in which they recorded the number of home exercise sessions they completed, as well as adverse events. They were also permitted to telephone or visit the physiotherapist if more advice was needed. The EG exercise programmes are shown in Appendix SI1.
Baseline (2 months post-operatively). The programme consisted of isometric strengthening exercises for the quadriceps and hamstrings muscles at multiple knee joint angles, performed in a sitting position. In the functional exercises, the participant’s body weight was used as resistance: rising on the toes, first on both legs and then on one leg. In the step exercises, a step height of 20 cm, which is the standard step height of stairs, was used. At the beginning, the participants were instructed to perform 2 series of 10 repetitions daily. They were instructed to increase the number of repetitions in each successive set by 1 or 2, up to a maximum of 20 repetitions and then to increase the number of series to 3. The physiotherapist telephoned the participants after the first 2 training weeks to ensure that there were no adverse events or any problems with the programme.
One-month check-up visit (3 months post-operatively). The new exercise programme included the following: (i) squats; (ii) hack squats with the back held against the wall; and (iii) step exercise with a 30 cm high gym bench. In both of the squat exercises, a 60º knee angle in the down position was used. The participants started performing 15 repetitions with 1–2 kg dumbbells in both hands for additional resistance. When the participants were able to perform 20 repetitions and 3 sets, they were then requested to increase the weight of the dumbbell by 1–2 kg. The participants had to purchase their own dumbbells for use at home. They were instructed to continue exercising 3 times per week.
Four-month visit (6 months post-operatively). The progressions of the previously used exercises (i–iii) were increased. In the squat and hack squat exercises, the participants were instructed to increase the knee angle from 60º to 90º. In the squat exercise, the participants were instructed to increase the load on the operated side by shifting the body weight more to the operated leg by placing a book under the opposite leg. Participants were recommended to exercise 3 times per week, and they were encouraged to continue training up to the 12-month follow-up. They were provided with postage-paid envelopes to return exercise diaries on a monthly basis.
Flexibility exercises. These consisted of hamstring and triceps surae stretching exercises in a standing position, and quadriceps and hip flexor stretching in a prone position. Each stretch was to be held for 30 s with 5 repetitions, and it was to be performed after strength training.
Control group. The CG did not receive any additional guidance after the baseline measurements, in accordance with normal care.
Outcomes
The participants completed a questionnaire to obtain demographic characteristics and health and work status. Weight and height were measured to calculate body mass index (BMI). Disability was assessed with the WOMAC questionnaire, which consists of 3 subscales: pain (5 questions); stiffness (2 questions); and functional difficulties (17 questions). Each question was graded on a visual analogue scale, ranging from 0 to 100 mm, with 100 indicating the worst possible situation (18). The Finnish version of the WOMAC questionnaire has been validated previously (19).
HRQoL was measured using the Short Form-36 Health Survey (SF-36), which contains 8 dimensions: physical functioning (PF); physical role (RP); bodily pain (BP); general health (GH); vitality (VT); social functioning (SF); emotional role (RE); and mental health (MH). Each subscale is scored from 0 to 100, with higher scores indicating better health status. In addition, the 8 scales of the SF-36 were aggregated into 2 summary scores: the Physical Component Summary Score (PCS), comprising PF, RP, BP and GH; and the Mental Component Summary Score (MCS), comprising VT, SF, RE and MH (20).
The Timed Up and Go test (TUG-test) was used to measure basic mobility (21). The interclass correlation coefficients of the TUG test has been reported to be 0.80 among TKA patients (22).
Active and passive knee range of motion (ROM) were measured using a standard, plastic long-arm goniometer with the subject positioned in the supine position (23). In a recent study, the ICC of a long-arm goniometer when measuring the active and passive ROM of a knee joint was reported to vary between 0.89 and 0.97 (24).
Maximal walking speed without shoes was measured using the GAITRite Walkway System (CIR Systems Inc., Sparta, USA). Participants were instructed to walk barefoot as rapidly as possible, but still safely. The participants started walking from a point 2 m in front of the mat, and stopped at a point 2 m beyond the mat. The ICC of the maximal walking speed, measured with the GAITRite Walkway System used here, was reported to be 0.89 (25).
The isometric knee flexion and extension strength levels were measured using a fixed dynamometer (Ds Europe, mod. 546QTD strain gauge, Milan, Italy) (5). During measurements, the participants were seated with the knee and hip joints at 70º flexion, and a security strap was placed over the pelvis to prevent use of the body muscles. After 3 submaximal repetitions for warm-up, the participants were instructed to perform 3 maximal muscle contractions with a 1-min rest period following each effort. If the third performance improved by more than 5% over the best result, an additional trial was performed. The best result of each measurement was used in the final analysis. The ICC of isometric strength measurements was reported to be 0.96 (26).
Statistical analysis
Statistical analysis was performed according to intention to treat (ITT) principles. The data are presented as means with standard deviations (SD) and totals with percentages. Ninety-five percent confidence intervals (95% CIs) are reported for the most important outcomes. Comparisons between the groups were performed using the t-test, the χ2 test, or analysis of covariance (ANCOVA), with the baseline values as covariates. Repeated measures for continuous outcomes were analysed using a mixed model approach, with the unstructured correlation structure data for an appropriate contrast. Hochberg’s approach for multiple comparisons was applied to correct levels of significance for multiple testing, if appropriate (27).
Sample-size estimation. The intended sample size was based on the primary hypothesis (WOMAC subscale for pain). Assuming a mean difference of 6 in change in WOMAC pain score between the groups at 12 months (SD 10), a sample size of 100 (50 in each group) was required to detect an effect size of 0.50 at alpha of 0.05 and power of 85%. The drop-out rate was estimated to be 10%.
RESULTS
The preoperative demographic and clinical characteristics of the groups are shown in Table I. There were no differences between the groups, except that the duration of pain in the operated knee was longer in the CG.
|
Table I. Preoperative demographic and clinical characteristics of the study population |
||
|
Variable |
Group |
|
|
Exercise (n = 53) |
Control (n = 55) |
|
|
Demographic characteristics Female, n (%) Age, years, mean (SD) Body mass index, kg/m2: mean (SD) Employed, n (%) Education, years, mean (SD) Family status, cohabitating, n, (%) |
30 (57) 69 (8) 31 (5) 9 (17) 9 (4) 34 (64) |
36 (65) 69 (9) 31 (6) 9 (16) 9 (3) 35 (64) |
|
Clinical characteristics Earlier knee operations, n (%) operated knee arthroscopy osteotomy knee arthroplasty contralateral knee arthroscopy osteotomy knee arthroplasty Duration of knee pain, months (SD) General health Comorbidity, n (%) cardiovascular disease diabetes neurological disease pulmonary disease other chronic disease |
27 (51) 6 (11) 0 (0) 14 (26) 2 (4) 15 (28) 66 (61) 12 (23) 11 (21) 2 (4) 7 (13) 6 (11) |
23 (42) 3 (5) 0 (0) 11 (20) 6 (11) 17 (31) 109 (104) 12 (22) 8 (15) 1 (2) 5 (9) 5 (9) |
|
SD: standard deviation. |
||
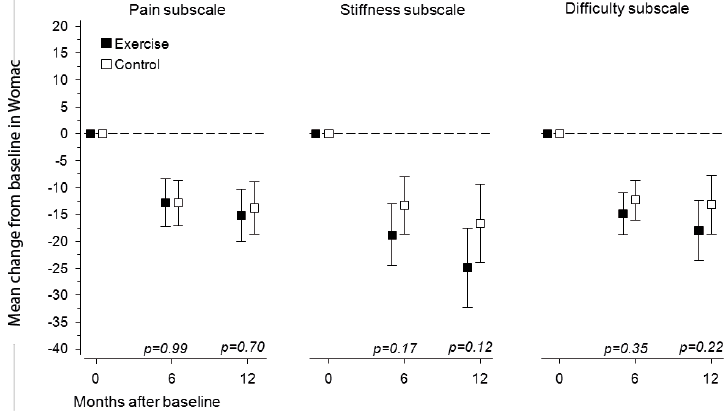
At baseline, the WOMAC subscale scores for pain (EG 23 [SD 18] vs CG 23 [SD 18]) stiffness (EG 37 [SD 24] vs CG 37 [SD 26]) and functional difficulty (EG 26 [SD 20] vs CG 27 [SD 20]) did not differ between the groups. In the EG and CG, the mean decreases in the respective subscales were –15 (95% CI –20 to –10) and –14 (95% CI –19 to –9) for pain, –25 (95% CI –32 to –18) and –17 (95% CI –24 to –9) for stiffness, and –18 (95% CI –24 to –12) and –13 (95% CI –19 to –8) for functional difficulty (Fig. 2). In both groups, the mean changes in all of the WOMAC subscales scores were statistically significant (p < 0.001), but no significant differences between the groups were found.
Fig. 2. Changes in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) subscale scores from baseline to 12-month follow-up. Boxes show means, and whiskers show 95% confidence intervals.
At baseline, the PCS of the SF-36 (EG 31 [SD 7] vs in CG 33 [SD 7]) and MCS of the SF-36 ([EG 51 [SD 11] vs CG 51 [SD 11]) did not differ between the 2 groups. The mean changes of 8 (95% CI 6 to 11) in the EG participants and 6 (95% CI 4 to 9) in the CG in PCS (p < 0.001) and those of 4 (95% CI 1 to 7) in the EG and 3 (95% CI 0 to 6) in the CG in MCS were statistically significant (p < 0.001), with no between-group differences at any time-point (Fig. 3).
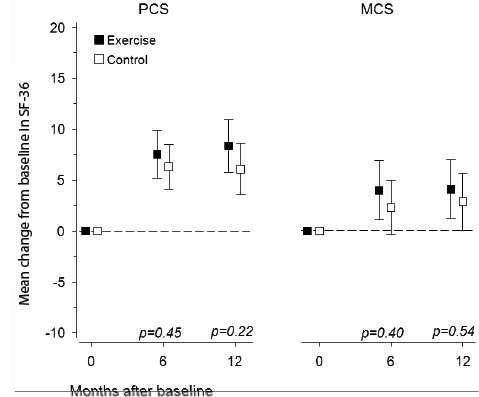
Fig. 3. Changes in the Physical (PCS) and the Mental (MCS) Component Summary Scores of the Short Form-36 questionnaire (SF-36) from baseline to 12-month follow-up. Boxes show means, and whiskers show 95% confidence intervals.
With regard to the physical performance variables, the EG improved statistically significantly in knee flexion strength and maximal walking speed, compared with the CG (Table II). Both groups improved significantly in knee extension strength, TUG test time and the ROM of the operated knee, but no significant differences between the groups were found. After adjustment for multiple comparisons, a statistically significant difference between the groups was found in maximal walking speed (p = 0.0064).
|
Table II. Outcome measurements of physical performance at baseline (2 months postoperatively) and after 12 months of training |
|||||||
|
Baseline |
Changes from baseline to 12 months after training |
p-value between groupsa |
|||||
|
Exercise group Mean (SD) |
Control group Mean (SD) |
Exercise group Mean (95% CI) |
Control group Mean (95% CI) |
||||
|
Isometric strength of operated knee, kg Extension Flexion |
18.2 (8.9) 10.3 (4.7) |
14.8 (7.8) 10.1 (5.1) |
15.1 (12.5 to 17.6) 4.4 (3.1 to 5.7) |
13.1 (10.1 to 16.1) 2.4 (1.3 to 3.4) |
0.50 0.009 |
||
|
Maximal walking speed, m/s |
1.36 (0.44) |
1.35 (0.37) |
0.32 (0.26 to 0.38) |
0.17 (0.11 to 0.24) |
< 0.001 |
||
|
Timed Up and Go test, s |
9.5 (2.8) |
10.5 (3.6) |
–1.58 (–2.53 to –0.63) |
–1.43 (–2.01 to –0.85) |
0.16 |
||
|
Range of motion of operated knee, degree Passive extension deficit Active extension deficit Passive flexion Active flexion |
5.0 (5.1) 9.0 (5.0) 104.0 (13) 99.0 (13) |
4.3 (4.9) 8.3 (4.6) 103.0 (17) 100.0 (17) |
–3.7 (–5.1 to –2.4) –5.9 (–7.3 to –4.5) 13.2 (10.6 to 16.1) 14.4 (11.5 to 17.4) |
–3.5 (–4.6 to –2.4) –6.0 (–7.0 to –5.0) 13.9 (10.1 to 17.7) 14.2 (10.1 to 18.1) |
0.72 0.45 0.86 0.98 |
||
|
aAnalysis of covariance; baseline value as covariate. SD: standard deviation; 95% CI: 95% confidence interval. |
|||||||
Compliance and safety
During the first 6 months, 72% of the participants in the EG performed the planned training sessions at least twice per week according to the training diaries. After that period, the training diary data were insufficiently complete to be included in any analysis. According to the 12-month questionnaire, 49% of the EG and 34% of the CG performed exercises at least once per week during the last month (p = 0.24). For those who had been exercising regularly, the mean exercise frequency was 3.5 (SD 2.2.) times per week in the EG and 4.2 (SD 2.6.) times per week in the CG (p = 0.40). More participants in the CG received additional advice for knee exercises in a local healthcare centre or in a private physiotherapy service, compared with the EG (38 vs 17%, p = 0.008). Participants in both groups reportedly engaged in leisure-time physical activities for a similar amount of time, mean 4.7 (SD 3.5) times per week (EG) vs 4.0 (SD 2.5) times (CG) (p = 0.55).
In the EG, 5 participants discontinued the training due to pain during exercising: 2 participants reported knee pain on the operated side, 1 reported pain on the contralateral side, 1 reported back pain, and 1 reported hip pain. In addition, 5 discontinued training because they were satisfied with their painless knees and were no longer motivated to engage in training. One participant was re-operated on due to a reduced range of motion, which was considered to be caused by arthrofibrosis. All of these participants were included in the intention-to-treat analysis.
DISCUSSION
The long-term home exercise programme in this study improved physical performance by increasing maximal walking speed and knee flexion strength significantly more in the EG compared with the CG. Both groups showed marked improvements in self-reported pain, disability and HRQoL, but no differences were found between the groups.
During the intervention, which commenced 2 months postoperatively, the WOMAC pain, disability and stiffness subscale scores decreased in both groups, but no between-group differences were observed, either at 6 or 12 months after baseline. Also, in the home-based exercise study by Kramer (10), no differences between the intervention groups were found in any subscale scores of WOMAC assessed 12 months after surgery. Kramer et al. (10) compared home-based and clinically based exercise programmes implemented between weeks 1 and 12 after TKA. One difference between the present study and the study by Kramer et al. (10) was that, in the present study, the intervention started 2 months after surgery, while the other study started 2 weeks postoperatively.
In previous studies in which an intervention was started 2 months post-surgery, as in the present study, and lasted 3–12 weeks, no differences were found between the training and control groups in any WOMAC subscale scores over 6 or 12 months of follow-up (14, 15, 28). However, Moffet (28) reported that a supervised exercise programme decreased WOMAC pain and functional difficulty subscale scores significantly more in the intervention group than in the normal care group immediately following the intervention, which lasted 2 months longer. However, these differences disappeared by the 12-month follow-up. Kauppila et al. (15), comparing normal care and multidisciplinary rehabilitation after surgery, did not show between-group differences in any of the WOMAC subscales 12 months after surgery. Piva et al. (14) compared 2 different 6-week functional training programmes and found no significant differences in WOMAC pain or stiffness subscales scores at 6 months of follow-up.
Both groups in the present study improved similarly in HRQoL. Accordingly, in the study by Kramer (10), HRQoL, measured using SF-36, improved similarly in both the home-based and in-patient rehabilitation groups over a 12-month follow-up. Moffet et al. (28) found small, but significant, differences in favour of the intensive functional rehabilitation group in the PCS and MCS scores of the SF-36, compared with usual care group after a 2-month intervention period, but the differences disappeared during the 12 months of follow-up. Kauppila et al. (15) found no significant difference in HRQoL measured using 15D at any follow-up point.
Despite the extended home programme not yielding greater improvements in WOMAC pain or HRQoL, it did have some positive effects on physical performance. Faster maximal walking speed was found in the EG than in the CG. This observation was meaningful because walking is a basic human function, and limitations in walking increase the risk for disability (29) and dependency (30), and reduce social networks (31). Normal walking speed among persons aged 60–69 years has been found to be a mean of 1.29 m/s, which is approximately 30% slower than maximal speed (32, 33). To cross at a light-controlled crossing requires a walking speed of approximately 1.2 m/s (34), which is thus the speed required to cope as a pedestrian in ordinary traffic conditions. At baseline, more than one-third of the participants in both groups did not reach that speed, even if they walked at maximal speed. At the 12-month follow-up, more than 90% of the participants in the EG reached 1.2 m/s in walking speed, while in the CG, the corresponding proportion was 79%.
Moreover, the home exercise programme yielded greater knee flexion strength in the EG, compared with controls. Greater knee muscle strength and knee motion have been reported to have a positive effects on balance (35) and on the prevention of falling (36). In the present study, the CG also gained knee muscle strength, which might be partly explained by the advice received from local healthcare centres or from private physiotherapy practices or by the increase general physical activity. Valtonen et al. (37) also found that, in the exercise group, while the benefits achieved in knee flexor and extensor muscle power were maintained, the mobility benefits (habitual walking speed and stair ascending time) disappeared at 12 months after the cessation of training. They concluded that habitual physical activity was sufficient to maintain muscle power in sedentary people. In contrast, in the study of Bade et al. (38), stair climbing and walk test results remained better in the high-intensity programme group than in the low-intensity programme group at the 52-week follow-up. In their study, the training period started immediately after TKA and lasted 8 weeks, and even high-intensity resistance and eccentric training was well tolerated. In the present study, the training programme started 2 months after the operations, because we wanted to allow for early healing of wounds and soft tissue so that knee pain, effusion or movement limitations would not complicate the training (28).
The long-term home programme had a low cost and appeared to be well tolerated, as only 2 participants reported pain in the operated knee, and no major complications were reported. The programme consisted of functional exercises, which are easy to perform progressively. The participants were instructed to exercise 3 times per week. According to the training diaries, during the first 6 months, 72% of the participants exercised at least twice per week, which has been proposed to be sufficient to increase strength (16). Over the next 6 months, training compliance declined, and the strength increase was consequently less than expected. Five participants discontinued the exercise programme due to pain and 5 because they were satisfied with their painless knees and were no longer motivated to train. To improve compliance, it might be possible in clinical practice to offer modified exercises, such as water gymnastics or treatment for pain. It is more difficult to improve exercise compliance in participants who are satisfied with the results of their surgery and thus are not motivated to persist with long-term training. In shorter interventions and in supervised training studies, training compliance has been easier to maintain (14, 28).
The strength of the present randomized controlled study was the long training period, compared with the 3–12 weeks used in earlier intervention studies (12, 14, 15, 28). The study also had only a few drop-outs. According to the 12-month questionnaire responses, exercise compliance decreased considerably during the last month, and the training diaries were insufficiently completed during this period. Thus, a limitation of the study was poor exercise compliance. A home-based exercise programme might require a larger number of booster contacts to promote better exercise adherence and progression (39, 40). Participants with pain might need effective therapies to abolish pain in order to increase their exercise compliance. In the present study, several participants received exercise guidance from other healthcare providers, and more often in the control group, which might have had influence on the results. Other limitations include lack of local reliability data for the objective tests.
In conclusion, a 12-month home-based exercise programme, which commenced 2 months after TKA, did not improve index knee pain beyond the improvement achieved by usual care. However, the exercise programme had some positive effects on objectively measured physical performance. The exercise programme was suitable for use at home for most participants and was easy to implement in general practice. However, means to increase exercise adherence must be further developed.

1http://www.medicaljournals.se/jrm/content/?doi=10.2340/16501977-1242.
ACKNOWLEDGEMENTS
This study was supported in part by a grant from the Central Finland Health Care District. The authors have no conflicts of interest to declare.
References
1http://www.medicaljournals.se/jrm/content/?doi=10.2340/16501977-1242.
