Sang Seok Yeo, PhD and Sung Ho Jang, MD
From the Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University, Daemyungdong, Namku, Taegu, Republic of Korea
OBJECTIVE: Knowledge about recovery of an injured fornix following brain injury is limited. We describe here a patient who showed recovery of an injured fornix following stroke.
CASE REPORT: A 57-year-old female patient underwent coiling for a ruptured anterior communicating cerebral artery aneurysm, and conservative management for subarachnoid and intraventricular haemorrhage. The patient showed severe cognitive impairment 6 weeks after onset. However, her cognition showed continuous improvement with time; based on the Mini-Mental State Examination and the Memory Assessment Scale, her cognition was within the normal range 7 months after onset.
RESULTS: Findings from diffusion tensor tractography at 6 weeks and 7 months showed discontinuations in both columns of the fornix. The proximal portion of both crus also showed discontinuation on diffusion tensor tractography at 6 weeks and 7 months; however, on 7-month diffusion tensor tractography, the end of the fornical body was shown to be connected to the splenium of the corpus callosum and then branched to the right medial temporal lobe and right thalamus.
CONCLUSION: The unusual neural connection between the injured fornix and the thalamus appears to be a recovery phenomenon, which allows the injured fornix and the medial temporal lobe to obtain cholinergic innervation from cholinergic nuclei in the brainstem rather than from cholinergic nuclei in the basal forebrain.
Key words: fornix; memory; diffusion tensor imaging; brain plasticity; stroke.
J Rehabil Med 2013; 45: 1078–1080
Correspondence address: Sung Ho Jang, Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University 317-1, Daemyungdong, Namku, Taegu, 705-717, Republic of Korea. E-mail: strokerehab@hanmail.net
Accepted Aug 30, 2013; Epub ahead of print XXX ?, 2013
Introduction
The fornix is an important structure of the Papez circuit through which information on episodic memory is transferred between the medial temporal lobe and the medial diencephalon. Until recently, accurate assessment of the fornix using conventional brain computerized tomography (CT) and magnetic resonance imaging (MRI) has been difficult due to its long, thin shape, and its location deep within the brain (1). By contrast, diffusion tensor tractography (DTT), derived from diffusion tensor imaging (DTI), allows for 3-dimensional visualization and estimation of the fornix (2). Many studies using DTT and DTI have demonstrated injury of the fornix in various brain pathologies, including intracerebral haemorrhage, intraventricular haemorrhage, traumatic brain injury, subarachnoid haemorrhage, encephalitis, and schizophrenia (3–7). As a result, the fornix is known to be vulnerable to brain injury. However, knowledge about recovery of an injured fornix after brain injury is limited (8).
In this study, using follow-up DTT, we report on a patient who showed recovery of an injured fornix following stroke.
Case report
One patient and 8 age-matched control subjects (2 men, 6 women; mean age 52.1 years, age range 48–60 years) with no history of neurological disease were recruited for this study. The patient, a 57-year-old, right-handed woman underwent coiling for a ruptured anterior communicating cerebral artery aneurysm and conservative management for subarachnoid and intraventricular haemorrhage at the neurosurgery department of a university hospital (Fig. 1A). Six weeks after onset, she was transferred to the rehabilitation department of the same university hospital for rehabilitation of her poor cognition and quadriparesis. Brain MRI 6 weeks after onset showed white matter damage in the basal forebrain and left medial frontal area (Fig. 1A). The patient underwent a comprehensive rehabilitative management programme, including movement therapy, cognitive therapy, and medication choline alfoscerate 3 T/day (maximum dosage: 3 T/day), dopaminergic drugs: ropinirole 2 mg/day (24 mg/day), amantadine 200 mg/day (400 mg), and levodopa 375 mg/day (750 mg)). All participants provided written informed consent prior to the study, and the institutional review board of a university hospital approved the study protocol.
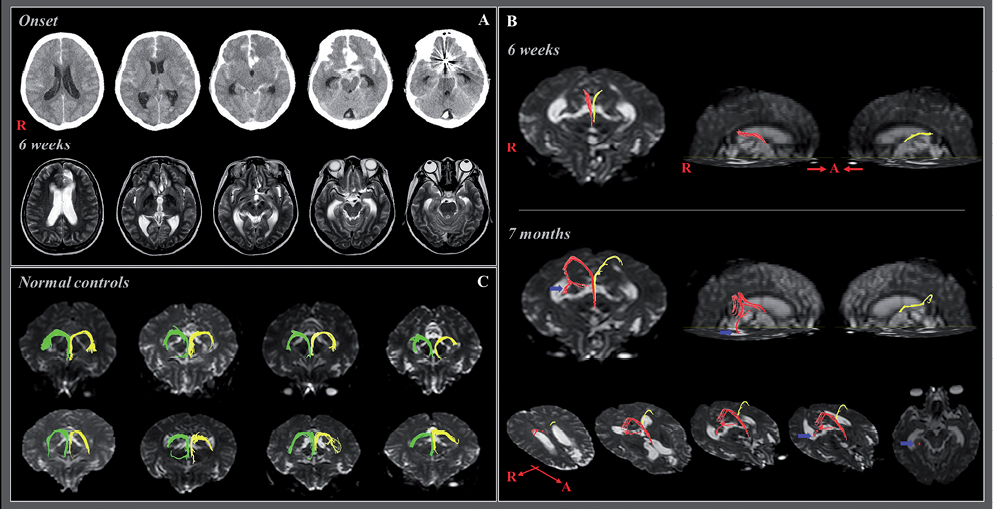
Fig. 1. (A) Brain computerized tomography in a female stroke patient on the day of onset after coiling shows a subarachnoid haemorrhage, intraventricular haemorrhage, and intracerebral haemorrhage in the basal forebrain. T2-weighted magnetic resonance images taken at 6 weeks after onset of white matter damage in the basal forebrain and left medial frontal area. (B) Diffusion tensor tractography (DTT) of the fornix. Discontinuations in both columns of the fornix were observed on 6-week and 7-month DTTs. The proximal portion of both crura also showed discontinuations on 6-week DTT; however, the end of the fornix body was connected to the splenium of the corpus callosum and then branched to the right medial temporal lobe (blue arrow) and right thalamus. (C) None of these abnormal neural tracts was observed in 8 age-matched normal control subjects.
Clinical evaluation
Three scales were used for evaluation of cognitive function; the Mini-Mental State Examination (MMSE) (9), the Wechsler Intelligence Scale (10), and the Memory Assessment Scale (MAS) (11). The patient showed severe cognitive impairment at 6 weeks after onset, as 11 on the MMSE (full mark 30); her cognition could not be evaluated using the Wechsler Intelligence Scale and MAS. However, her cognition showed continuous improvement over time; consequently, cognition on the MMSE, the Wechsler Adult Intelligence Scale, and the MAS were within the normal range at 7 months after onset (MMSE: 30, total IQ: 108, global memory: 118 (89%ile), short term memory: 101 (53%ile), verbal memory: 117 (87%ile), and visual memory: 116 (86%)).
Diffusion tensor imaging
DTI was performed twice (6 weeks and 7 months after onset) using a 1.5-T Philips Gyroscan Intera system (Philips, Best, The Netherlands) equipped with a Synergy-L Sensitivity Encoding (SENSE) head coil using a single-shot, spin-echo planar imaging pulse sequence. For each of the 32 non-collinear and non-coplanar diffusion sensitizing gradients, we acquired 60 contiguous slices parallel to the anterior commissure – posterior commissure line. Imaging data were acquired from the area between the cortex and the middle of the second cervical vertebral body. Imaging parameters were as follows: acquisition matrix = 96 × 96, reconstructed to matrix = 192 × 192 matrix, field of view = 240 × 240 mm2, TR = 10,726 ms, TE = 76 ms, parallel imaging reduction factor (SENSE factor) = 2, EPI factor = 49, b = 1000 s/mm2, NEX = 1, and slice thickness 2.5 mm (acquired isotropic voxel size 2.5 × 2.5 × 2.5 mm3). Affine multi-scale 2-dimensional registration was used for reduction of eddy current-induced image distortions and motion artefacts. Pre-processing of DTI data-sets was performed using the Oxford Centre for Functional Magnetic Resonance Imaging of Brain (FMRIB) Software Library (FSL). DTI-Studio software (CMRM, Johns Hopkins Medical Institute, USA) was used for reconstruction of the CRP and CST. For analysis of the CRP of the fornix, we placed a seed region of interest (ROI) at the junction between the body and column of each fornix on a coronal image of the colour map. Target ROIs were placed on the crus of the right and left fornix on a coronal image of the colour map, respectively (7). The termination criteria applied were a fractional anisotropy (FA) of < 0.2 and an angle change of > 70°.
Discontinuations in both columns of the fornix were observed on 6-week and 7-month DTT. Discontinuations in the proximal portion of both crus were also observed on 6-week DTT; however, observations showed that the end of the fornical body was connected to the splenium of the corpus callosum, and branched to the right medial temporal lobe and right thalamus (Fig. 1B). This connection was not observed in 4 normal control subjects (Fig. 1C).
Discussion
In the current study, we investigated changes in DTT findings of an injured fornix in a patient who had had a subarachnoid haemorrhage and an intraventricular haemorrhage. Findings on 6-week DTT showed discontinuation of the bilateral fornix crura; however, on 7-month DTT, an unusual neural tract connecting the injured fornical body to the right medial temporal lobe and thalamus via the splenium of the corpus callosum was observed. Previous studies have demonstrated the vulnerability of the fornix to subarachnoid haemorrhage, intraventricular haemorrhage, and intracerebral haemorrhage (6, 7). As a result, it appears that the fornix injury in this patient was ascribed to the subarachnoid haemorrhage and intraventricular haemorrhage. Direct connection of the fornical crus to the medial temporal lobe in the normal human brain has been reported; however, no unusual neural tract connecting the fornical body to the right medial temporal lobe and thalamus via the splenium of the corpus callosum was observed in normal control subjects or in previous studies (2). We believe that this unusual neural tract of the fornix is the result of development of compensatory neural tracts following injury of the fornical crus. The fornix is known to obtain cholinergic innervation from the medial septal nucleus and vertical nucleus of the diagonal band in the basal forebrain (12, 13). However, several studies have reported that the thalamus is a target area of cholinergic nuclei (the pedunculopontine nucleus and laterodorsal tegmental nucleus) in the brainstem (12, 14, 15). The unusual neural connection between the injured fornix and the thalamus appears to be a recovery phenomenon, which allows the injured fornix and the medial temporal lobe to obtain cholinergic innervation from cholinergic nuclei in the brainstem rather than from cholinergic nuclei in the basal forebrain. We believe that the existence of this unusual connection may suggest a possible recovery phenomenon between cholinergic nuclei and the Papez circuit following brain injury. Previous studies using DTI have reported poor memory function in patients with fornix injury after brain injury (5–7). The good memory function observed in this patient may be additional evidence of the patient’s recovery.
In conclusion, we report here a case of a stroke patient who appeared to show recovery of an injured fornix. This finding appears to suggest a mechanism for recovery of an injured fornix following brain injury. To the best of our knowledge, only a few studies have reported on the recovery of an injured fornix in patients with brain injury (8). In a recent report, a patient showed an abnormal neural tract originating from the injured fornix passing through the splenium of the corpus callosum to connect with the inferior longitudinal fasciculus following traumatic brain injury (8). This study also suggested that such unusual neural tracts originating from the injured fornix were the result of development of compensatory neural tracts following bilateral injury of the fornix crus due to traumatic brain injury. Therefore, this is the first study to report on the recovery of an injured fornix in stroke patients.
The limitations of DTI should be considered (16). DTI is a powerful anatomical imaging tool that can demonstrate gross fibre architecture. However, DTI may underestimate or overestimate the fibre tracts. In addition, regions of fibre complexity and crossing can prevent full reflection of the underlying fibre architecture by DTI (16). In addition, this study is limited as it is based on a single case report; thus further larger scale studies are required.
Acknowledgement
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A4A01001873).
References
