Margaret K. Y. Mak, PhD1 and Mandy M. Auyeung, FHKCP2
From the 1Department of Rehabilitation Sciences, The Hong Kong Polytechnic University and 2Department of Medicine, Pamela Youde Nethersole Eastern Hospital, Hong Kong, China
OBJECTIVES: To examine whether the Mini-Balance Evaluation Systems Test (Mini-BESTest) independently predicts recurrent falls in people with Parkinson’s disease.
DESIGN: The study used a longitudinal cohort design.
SUBJECTS: A total of 110 patients with Parkinson’s disease completed the study and were included in the final analysis. Most of the patients had moderate disease severity.
METHODS: All subjects were measured to establish a baseline. The tests used were Unified Parkinson’s Disease Rating Scale (MDS-UPDRS III), Freezing of Gait Questionnaire, Five-Time-Sit-To-Stand Test, and Mini-BESTest. All patients were followed by telephone interview for 6 months to register the incidence of monthly falls.
RESULTS: Twenty-four patients (21.2%) reported more than one fall and were classified as recurrent fallers. Results of the multivariate logistic regression showed that, after adjusting for fall history and MDS-UPDRS III score, the Mini-BESTest score remained a significant predictor of recurrent falls. We further established that a cut-off Mini-BESTest score of 19 had the best sensitivity (79%) for predicting future falls in patients with Parkinson’s disease.
CONCLUSION: The results indicate that those with a Mini-BESTest score < 19 at baseline had a significantly higher risk of sustaining recurrent falls in the next 6 months. These findings highlight the importance of evaluating dynamic balance ability during fall risk assessment in patients with Parkinson’s disease.
Key words: accidental falls; balance; Parkinson’s disease; risk factors.
J Rehabil Med 2013; 45: 00–00
Correspondence address: Margaret K. Y. Mak, Department of Rehabilitation Sciences, Hong Kong Polytechnic University, Hung Hom, Hong Kong. E-mail: Margaret.Mak@inet.polyu.edu.hk
Accepted Jan 7, 2013; Epub ahead of print Apr 29, 2013
Introduction
Falls are one the most disabling features of Parkinson’s disease (PD). Up to 70% of individuals with PD experience a fall annually, and 25–50% fall twice or more in a year (1–4). Repeated falls can lead to devastating outcomes, such as functional limitations, physical deconditioning, increased chances of institutionalization and a higher mortality rate (5, 6). One large-scale prospective study on older adults showed that multiple fallers had significantly greater functional decline than single fallers (7). Therefore, repeated falls are a serious problem, and early identification of potential recurrent fallers is needed. Parkinsonian fallers have been shown to have poor balance ability; they have a lower Berg’s Balance Score (BBS) (8), shorter 1-leg stance time (9), poor leaning stability (10), more postural sway (2), and poorer Romberg tests (1) than their non-falling counterparts. Ideally, balance impairment could be used to predict future falls. However, in previous studies, only poor leaning stability combined with fall history, gait freezing, and knee muscle weakness were found to predict future falls in people with PD (10). It is possible that postural instability is multi-factorial and balance measures that evaluate one aspect of balance performance may not be sensitive enough to predict PD fallers.
Franchignoni et al. (11) have developed a new clinical tool called the Mini-Balance Evaluation Systems Test (Mini-BESTest). This is a comprehensive assessment tool that measures 4 different balance control systems: sensory organization, anticipatory postural adjustments, postural responses, and dynamic balance during gait. Previous studies have found that this test has excellent inter-rater reliability (intraclass correlation coefficient (ICC = 0.91) and test-retest reliability (ICC = 0.92) in patients with PD (8, 12). The Mini-BESTest has also been found to have less of a ceiling effect than the BBS (13), and it can identify potential fallers in individuals with PD (8, 12). However, the fall incidences in these studies were reported retrospectively by patients with PD, which could have induced a recall bias. Duncan et al. (14) recently reported that the Mini-BESTest score was a significant predictor of PD fallers, with a higher accuracy of prediction in the 6-month follow-up period than in the 12-month period. However, these investigators did not include other potential fall risk factors in their prediction model. The aim of this study was to examine whether the Mini-BESTest could independently predict recurrent Parkinsonian fallers after accounting for other potential fall risk factors including demographic data (age, gender, PD duration, PD severity), PD-specific impairment, gait freezing, and muscle weakness.
Methods
Subjects
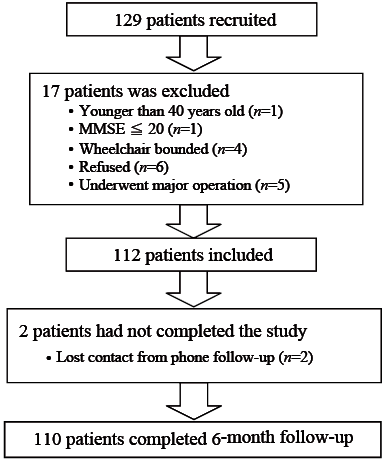
A total of 110 patients with PD completed the study (Fig 1). Subjects were recruited from the Hong Kong Parkinson’s Disease Association, a patient self-help group, and from Movement Disorders Clinics. Posters were sent to the Association and clinics, and patients were invited to join the study on a voluntary basis. Subjects were included if they were between 40 and 85 years old, had a diagnosis of idiopathic PD according to the UK PD Society Brain Bank Criteria (15), were medically stable, community-dwelling and could independently walk a minimum distance of 7 m, 3 times with or without walking aids. Participants were excluded if they had neurological conditions other than PD; communication deficits or cognitive impairment (Mini-Mental State Examination; MMSE < 20) (16); postural hypotension; visual or vestibular dysfunction; or significant cardiovascular or musculoskeletal disorders that affected balance and locomotion. A total of 129 patients volunteered for the study. Of these, 17 were excluded for the reasons stated in Fig. 1. Thus, 112 patients were eligible for the study, but 2 were excluded during the follow-up period due to lost contact. Ethics approval was obtained from ethics committees of the Hong Kong Polytechnic University and Hospital Authority Hong Kong East Cluster. Informed written consent was provided by all subjects in accordance with the 1964 Declaration of Helsinki.
Fig. 1. Flow chart of the selection process of individuals with Parkinson’s disease. MMSE: Mini-Mental State Examination.
Procedures
All assessments were tested during the “on” phase of the medication cycle, i.e. within 2 h of the subjects taking their anti-Parkinsonian drugs. Most of the subjects were assessed at the university’s gait and motion research laboratory. Twenty-five subjects were assessed in Hong Kong Parkinson’s Disease Association centres. Each subject underwent a baseline evaluation of the following outcomes.
Baseline measurement. Demographic data, including age, medication, time since diagnosis of PD and the number of falls in the previous 12 months, were recorded. The subjects were classified as having a positive fall history if they had had at least one fall in the past 12 months. A fall was defined as an event during which a subject comes to rest on the ground or at some lower level, not as the result of a major intrinsic event, e.g. syncope, stroke and seizure, or overwhelming hazard (17). Disease severity was assessed using the Hoehn and Yahr Staging scale (HY stage) (18). It consists of 7 stages ranging from stage 0 (no signs of disease) to stage 5 (wheelchair-bound or bedridden). The Movement Disorder Society’s revision of the Unified Parkinson’s disease rating scale (MDS-UPDRS) motor examination (III) was used to measure the PD-specific motor impairment and disability level of the subjects (19). Part III of this examination consists of 18 items with 33 questions pertaining to motor aspects of the disease, such as rigidity, tremor, bradykinesia, getting up from a chair, gait, posture, and postural stability. Each item is rated from 0 to 4, with a higher score indicating more severe motor impairment; the total score is 132.
The Chinese version of the Geriatric Depression Scale (GDS) was used to determine the level of depression in subjects (20). The score ranges from 0 to 15 with a higher score indicating more severe depression. A subject was classified as depressed if he/she obtained a GDS score higher than 6. The recent physical activity level of the subject was assessed with the Physical Activity Scale for the Elderly (PASE) (21). This questionnaire consists of 10 questions that assess the frequency and duration of an individual’s leisure, household and work-related activities in the past 7 days. The amount of time that an individual spends on each activity is categorized using both frequency and duration of the activity. The total PASE score is generated by multiplying the amount of time spent in each activity by item weighting and summing all the activities. PASE scores can range from 0 to 400, with a higher score indicating a higher physical activity level.
The Freezing of Gait Questionnaire (FOGQ) was used to detect and rate each individual’s subjective perception of the severity and impact of freezing on his/her gait performance (22). It consists of 6 items, with 4 that assess FOG severity and 2 that assess walking difficulties in general. FOGQ is rated on a 5-point scale from 0 to 4 and the total score ranges from 0 to 24. A higher score implies that the walking performance of the subject is more affected by freezing. The test-retest reliability (ICC = 0.84, p < 0.01) and internal reliability (Cronbach’s alpha = 0.89) of FOGQ were satisfactory (22).
The Five-Time-Sit-To-Stand Test (FTSTS) was used to examine the subjects’ functional lower-extremity muscle strength (23). These investigators have found FTSTS to be a quick measure for gross determination of fall risk in patients with PD (23). Subjects were instructed to cross their arms over their chest and to sit on a chair with their back against the back-support. During the test, the subjects had to, as quickly as possible, fully stand up and then sit down with their buttocks touching the chair. The time taken from the beginning of the test until the subjects had assumed the sitting position for the fifth time was recorded in seconds.
The balance performance of subjects was assessed with the Mini-BESTest (11). The Mini-BESTest includes 14 items representing 4 domains of dynamic balance: (i) anticipatory postural adjustments (items 1–3 consisting of sit-to-stand, rise to toes, stand on right and left leg); (ii) postural responses (items 4–6 consisting of compensatory stepping in 4 different directions); (iii) sensory orientation (items 7–9 consisting of stance with eyes open, foam surface with eye closed, inclined surface with eyes closed); and (iv) balance during gait (items 10–14 consisting of gait during change speed, head turns, pivot turns, obstacles, time “get up and go” with dual tasks). The Mini-BESTest items are rated on a 3-point scale from 0 to 2 and the total score ranges from 0 to 28 with a higher score indicating better balance performance.
After the baseline measurement, the subjects were instructed to complete a fall diary and were also contacted by telephone on a monthly basis to record all the falls in the 6-month follow-up period. A subject was classified as a recurrent faller (RF) if they had more than one fall within the 6-month follow-up period.
Statistical analysis
SPSS 16.0 was used to analyse the data in this study. The level of statistical significance was set at < 5% for all statistical tests. The between-group differences for the continuous variables, including age, duration of PD, UPDRS, GDS, MMSE, PASE, FTSTS, FOGQ, Mini-BESTest total score and the scores for each domain were analysed with independent t-tests. The ordinal variables (i.e. HY staging) were tested using the Mann-Whitney U test; the χ2 test was used to compare nominal variables including fall history and gender.
The association of recurrent falls with FTSTS, FOGQ and Mini-BESTest were first examined using univariate logistic regression analyses. The variables that showed significant association with recurrent falls were then entered into a multivariate logistic regression model so as to identify the significant predictors of recurrent falls in patients with PD. Demographic data (i.e. age, gender, duration of PD, UPDRS, prior fall history, HY staging and GDS) were entered into the model as model 1. The potential fall predictors (i.e. MDS-UPDRS III, FOGQ and FTSTS) were entered as model 2 and finally, the Mini-BESTest score was entered as model 3 to examine its predictive power. The likelihood-ratio, sensitivity, specificity and the percentage of correct classification for each model were presented. To determine the best model of fall prediction, the likelihood-ratio test was performed. In addition, predicted probabilities from logistic regression equation of each model were used to construct receiver operating characteristic (ROC) curves and the area under curve (AUC) were compared among the models using the Statistical Analysis System software (24). For the best model, ROC analysis was performed for every significant predictor of recurrent falls. The optimal cut-off score was determined from the ROC curve based on the best overall sensitivity and specificity. Bootstrap approach was used to determine the confidence interval (CI) for the optimal cut-off score (25). We drew 500 bootstrap samples and obtained the cut-off score for each sample using ROC analysis. After obtaining the mean and standard deviation of the bootstrap samples, the 95% CI was calculated as 1.96 × standard deviation (as an estimate of the standard error) of the 500 bootstrap values.
Results
A total of 110 subjects completed the study and were included in the final analysis. Of these, 24 (21.8%) experienced more than one fall within the 6-month follow-up period and were thus classified as RF. Table I shows that RF had significantly higher HY staging scales (p < 0.001), higher FOGQ scores (p = 0.005), significantly longer FTSTS times (p = 0.004) and significantly lower Mini-BESTest scores (p < 0.001) than N-RF. Therefore, at the baseline measure, RF had more advanced PD, more gait freezing, weaker lower limb muscles and poorer dynamic balance than N-RF. Moreover, we found that RF performed significantly worse than N-RF in 3 of the Mini-BESTest domains, specifically anticipatory postural adjustment (p = 0.016), postural responses (p < 0.001) and sensory orientation (p = 0.001) (Table I).
|
Table I. Subject characteristics |
||||
|
Variables |
Non-recurrent fallers (n = 86) |
Recurrent fallers |
Mean difference |
p-value |
|
Age, years, mean (SD) |
63.5 (9.3) |
62.2 (7.5) |
1.4 (–2.7 to 5.5) |
0.505 |
|
Gender, female, n |
34 |
10 |
– |
0.914 |
|
Fall history, n |
30 |
21 |
– |
< 0.001* |
|
Falls incidence in 6-month follow-up, n |
17 |
498 |
– |
< 0.001* |
|
MMSE score, 0–30, mean (SD) |
28.0 (2.3) |
27.8 (2.7) |
0.2 (–0.9 to 1.3) |
0.715 |
|
Duration of PD, years, mean (SD) |
6.7 (4.4) |
9.0 (6.2) |
–2.2 (–4.5 to –0.2) |
0.109 |
|
Hoehn and Yahr score (0–5)a, median (min–max) |
2.5 (1.0 to 4.0)a |
3.0 (2.0 to 3.0)a |
– |
< 0.001* |
|
MDS-UPDRS III, 0–132, mean (SD) |
24.9 (9.7) |
27.5 (9.4) |
–2.7 (–7.0 to 1.8) |
0.240 |
|
Geriatric Depression Scale score, 0–15, mean (SD) |
5.9 (3.8) |
7.4 (4.4) |
–1.5 (–3.3 to 0.3) |
0.094 |
|
PASE, mean (SD) |
93.0 (44.0) |
80.3 (60.1) |
12.8 (–9.2 to 34.6) |
0.251 |
|
FOGQ score, 0–24, mean (SD) |
8.9 (6.1) |
13.0 (7.0) |
–4.1 (–7.0 to –1.3) |
0.005* |
|
FTSTS time, s, mean (SD) |
15.7 (7.3) |
21.0 (9.7) |
–5.4 (–8.9 to –1.8) |
0.004* |
|
Mini-BESTest score, 0–28, mean (SD) |
20.6 (4.0) |
16.7 (4.6) |
3.8 (1.8 to 5.8) |
< 0.001* |
|
Anticipatory postural adjustments (0–6) |
4.7 (1.0) |
4.1 (1.1) |
0.6 (0.1 to 1.1) |
0.016* |
|
Postural responses, 0–6 |
3.3 (2.1) |
1.4 (1.8) |
1.9 (0.9 to 2.8) |
< 0.001* |
|
Sensory orientation, 0–6 |
5.2 (0.9) |
4.4 (1.1) |
0.8 (0.3 to 1.3) |
0.001* |
|
Balance during gait, 0–10 |
7.5 (1.4) |
7.0 (2.1) |
0.5 (–0.2 to 1.2) |
0.177 |
|
*p < 0.05. aMedian (minimum-maximum) in ordinal data (Hoehn and Yahr scale). CI: confidence interval; MMSE: Mini-Mental State Examination; MDS-UPDRS III: Movement Disorders Society Unified Parkinson’s Disease Rating Scale III; PASE: Physical Activity Scale for the Elderly; FTSTS: Five-Time-Sit-To-Stand Test; FOGQ: Freezing of Gait Questionnaire; Mini-BESTest: Mini-Balance Evaluation System Test. |
||||
The univariate logistic regression analysis showed that a lower Mini-BESTest score (p = 0.001, odds ratio (OR) = 0.815), a higher FOGQ score (p = 0.008, OR = 1.105) and a longer FTSTS time (p = 0.014, OR = 1.073) were significantly associated with recurrent falls in patients with PD (Table II).
|
Table II. Results of univariate logistic regression for Freezing of Gait Questionnaire, Five-Time Sit-To-Stand Test and Mini-Balance Evaluation System Test |
||||
|
Independent variables |
B |
SE |
Odds ratio |
p-value |
|
FOGQ |
0.100 |
0.037 |
1.105 |
0.008* |
|
FTSTS |
0.070 |
0.029 |
1.073 |
0.014* |
|
Mini-BESTest |
–0.204 |
0.060 |
0.815 |
0.001* |
|
*p < 0.05. FTSTS: Five-Time-Sit-To-Stand Test; FOGQ: Freezing of Gait Questionnaire; Mini-BESTest: Mini-Balance Evaluation System Test; SE: standard error. |
||||
A multivariate logistic regression analysis was then used to analyse the predictive power of the Mini-BESTest (Table III).
|
Table III. Results of multivariate logistic regression for risk factors for predicting recurrent falls in Parkinson’s disease |
|||||
|
Independent |
B |
SE |
Odds |
p-value |
|
|
Model 1 |
Age |
–0.027 |
0.034 |
0.973 |
0.426 |
|
Gender |
–0.594 |
0.612 |
0.552 |
0.331 |
|
|
Duration of PD |
0.061 |
0.053 |
1.063 |
0.246 |
|
|
HY |
1.736 |
0.702 |
5.677 |
0.013* |
|
|
Prior fall history |
2.367 |
0.715 |
10.665 |
0.001* |
|
|
GDS |
0.041 |
0.074 |
1.042 |
0.581 |
|
|
Model 2 |
Age |
–0.038 |
0.037 |
0.963 |
0.305 |
|
Gender |
–0.923 |
0.668 |
0.397 |
0.167 |
|
|
Duration of PD |
0.061 |
0.053 |
1.063 |
0.242 |
|
|
HY |
2.231 |
0.930 |
9.309 |
0.016* |
|
|
Prior fall history |
2.353 |
0.782 |
10.521 |
0.003* |
|
|
GDS |
0.018 |
0.081 |
1.018 |
0.824 |
|
|
MDS-UPDRS III |
–0.065 |
0.042 |
0.937 |
0.127 |
|
|
FOGQ |
0.000 |
0.050 |
1.000 |
0.992 |
|
|
FTSTS |
0.060 |
0.034 |
1.062 |
0.082 |
|
|
Model 3 |
Age |
–0.072 |
0.043 |
0.931 |
0.092 |
|
Gender |
–1.783 |
0.845 |
0.468 |
0.135 |
|
|
Duration of PD |
0.074 |
0.055 |
1.077 |
0.181 |
|
|
HY |
1.223 |
1.082 |
3.396 |
0.258 |
|
|
Prior fall history |
2.188 |
0.761 |
8.917 |
0.004* |
|
|
GDS |
–0.034 |
0.091 |
0.967 |
0.708 |
|
|
MDS-UPDRS III |
–0.099 |
0.050 |
0.906 |
0.048* |
|
|
FOGQ |
0.013 |
0.053 |
1.013 |
0.810 |
|
|
FTSTS |
0.034 |
0.037 |
1.034 |
0.368 |
|
|
Mini-BESTest score |
–0.287 |
0.117 |
0.750 |
0.014* |
|
|
*p < 0.05. GDS: Geriatric depression scale; HY: Hoehn and Yahr scale; FOGQ: Freezing of Gait Questionnaire; FTSTS: Five-Time Sit-To-Stand Test; MDS-UPDRS: Movement Disorders Society version of Unified Parkinson’s Disease Rating Scale; Mini-BESTest: Mini-Balance Evaluation System Test; SE: standard error. |
|||||
Table IV illustrates that, among the 3 multivariate models, model 3 had the highest level of sensitivity (0.625), specificity (0.953) and overall accuracy of classifications (86.4%). The results of the likelihood ratio test also indicated that model 3 was better than model 1 and 2 in fall prediction. However, there was a lack of significant difference in AUC among the models (Table IV, Fig. 2). Finally, for the multivariate model 3, after accounting for the demographic data, FOGQ scores and FTSTS times, fall history (p = 0.004) and MDS-UPDRS III (p = 0.046), the Mini-BESTest score remained a significant predictor (p = 0.014) of future recurrent falls in patients with PD (Table III). ROC curve was constructed to determine the optimal cut-off score in each predictor for identifying future recurrent fallers. The suggested Mini-BESTest cut-off score was 19 (95% CI = 16.9, 21.0), the AUC was 0.75 with a moderate sensitivity of 0.79 and specificity of 0.67 (Fig. 3). For MDS-UPDRS III, the AUC was 0.585 (p = 0.204) and no cut-off was determined.
|
Table IV. Likelihood ratio, area under curve (AUC), sensitivity, specificity and percent of correct classification for each multivariate model and comparisons of likelihood ratio and AUC among multivariate models |
||||||
|
–2 log likelihood |
df |
AUC |
Sensitivity |
Specificity |
% of correct classification |
|
|
Model 1 Model 2 Model 3 |
83.166 78.195 71.416 |
6 9 10 |
0.853 0.868 0.895 |
0.375 0.542 0.625 |
0.942 0.907 0.953 |
81.8 82.7 88.2 |
|
Likelihood ratio test |
AUC difference |
|||||
|
Difference in –2 log likelihood |
Critical χ2 |
p-value |
χ2 for AUC difference |
p-value |
||
|
Model 1 vs 2 Model 2 vs 3 Model 1 vs 3 |
0.742 14.758 23.500 |
7.82 3.84 9.49 |
0.033* < 0.001* < 0.001* |
1.278 1.318 2.419 |
0.258 0.250 0.119 |
|
|
df: degree of freedom. *p < 0.05. |
||||||

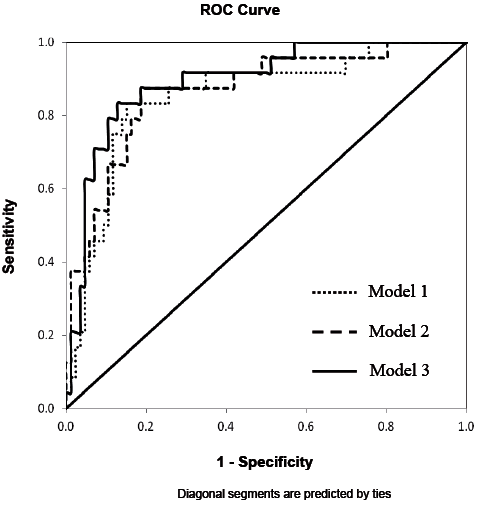
Fig. 2. Receiver operating characteristic (ROC) curves for multivariate models used to predict Parkinson’s disease recurrent fallers.
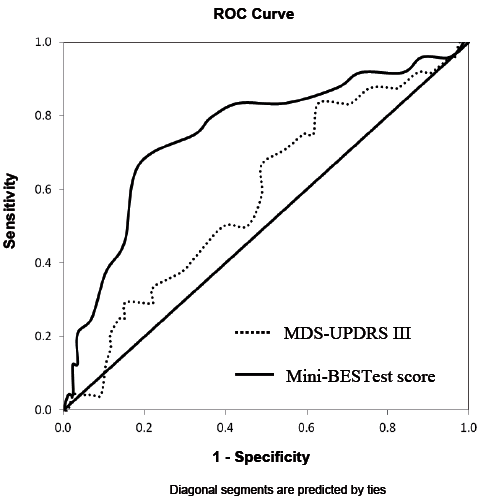
Fig. 3. Receiver operating characteristic (ROC) curves for determining the optimal cut-off score for the: Mini-Balance Evaluation System Test Mini-BESTest (Mini-BESTest) and Movement Disorders Society version of Unified Parkinson’s Disease Rating Scale (MDS-UPDRS III).
Discussion
This is the first prospective study to demonstrate that, after adjusting for prior falls, the Mini-BESTest score remains a significant predictor of future recurrent falls in patients with PD. Our results imply that poor dynamic balance ability significantly contributes to a higher risk of falling. The findings also suggest the potential role of balance enhancement programmes in the prevention of falls in patients with PD.
The recurrent fall rate in our study is 21.8%, which is comparable with previous studies (1, 9, 26). Previous studies reported that recurrent falls occurred in more than 50% of PD fallers (26, 27). In our study, 58.5% of the PD fallers experienced more than one fall in the follow-up period. Repeated falls result in many devastating adverse outcomes and substantial economic costs (6, 7) and it is imperative to identify fall predictors for recurrent fallers in individuals with PD.
Our study tested the 3 multivariate logistic regression models in an incremental way to demonstrate the independence of the Mini-BESTest in predicting RF. Findings of the likelihood ratio test demonstrates the superiority of model 3 in the prediction power. In addition, model 3 has the highest sensitivity, specificity and percent of correct classification, suggesting that adding Mini-BESTest score to model 3 increases the accuracy of identifying RF and non-RF. Despite these differences, the AUC is statistically equivalent for all models. All models include fall history, which is a strong predictor of future falls in individuals with PD (3, 4, 9, 10). A higher HY staging score has also been found to be a significant predictor of PD RF (1). The inclusion of these two parameters could have attributed to the large AUC even in the first regression model.
Our final multivariate model shows that MDS-UPDRS III is a significant RF predictor concurs with previous results (9, 26). We also find that a higher MDS-UPDRS score has an odds ratio of 0.905, suggesting that more severe motor impairment may restrict patients’ mobility and decrease their fall risk. Previous studies reported that gait freezing (10, 28) and reduced knee extensor strength (10) are fall predictors, but our results did not confirm this. FOGQ reflects an individual’s gait deficits and FTSTS evaluates a person’s lower extremity muscle strength. The Mini-BESTest, which documents both dynamic balance control and stability during complex gait activities, could be a more accurate fall prediction tool than a single test.
Among the Mini-BESTest domains, RF has significantly more deficits in anticipatory postural adjustment, postural response and sensory orientation, and these may have contributed to the increased risk of falling. Anticipatory postural adjustments are made by postural muscles that are activated in a feed-forward manner prior to an expected perturbation (29). People with PD have been shown to exhibit anticipatory postural adjustment with prolonged duration and reduced amplitude (30). An impaired postural preparation could increase the risk of falling during walking, turning, sit-to-stand transfer and leaning activities in standing (1, 10, 31). The commonly perceived causes of falls in individuals with PD are “trips and slips” during walking (27, 31). In response to these external perturbations, individuals with PD demonstrate longer latencies, shorter steps and slower step velocity in both lateral (32) and anterior-posterior directions (33). Individuals with PD also have difficulty in selecting appropriate postural response strategies to regain balance and in altering their response with a change in the direction of the perturbation (34). A poor postural response increases the risk of falling. With regards to sensory orientation, individuals with PD have impaired proprioceptive integration and rely more heavily on visual feedback when their equilibrium is challenged (33, 35). When blindfolded, individuals with PD and especially PD fallers might have difficulty using proprioceptive sensation to maintain their balance while standing on compliant and inclined surfaces, and would be predisposed to falls.
The lack of a between-group difference in the Mini-BESTest domain “dynamic gait stability” in our study is surprising, as walking and turning are the most common activities that cause falls (1, 27, 31). It is possible that both N-RF and RF experience difficulties in negotiating complex gait activities. For example, people with PD are unable to modulate their gait speed and to walk at a slow pace (36). Turning is difficult irrespective of whether the angle is small or large (37, 38) and dual-task walking is more difficult than walking alone (39, 40).
We further show that a Mini-BESTest with the cut-off score of 19 has a sensitivity of 0.79 and a specificity of 0.67. Duncan et al. (14) reported that a Mini-BESTest cut-off score of 20 had a higher sensitivity (0.86) and specificity (0.78) than ours. However, our results may be more valid than those of Duncan et al. (14) because our sample size is larger (110 vs 80) and the number of subjects lost during follow-up in our study is much smaller (2 vs 29). In addition, in applying the Mini-BESTest, we follow the scoring guideline of recording the lower performance of both the right and left sides for 2 items (stand on one leg and lateral stepping correction) on the Mini-BESTest. Duncan et al. (14) recorded both sides in these 2 items and reported a total score of 32 instead of 28. Their scoring method increases the weighting of these 2 items, which could affect the validity of the results. When compared with other outcomes, our reported sensitivity and specificity are higher than other clinical measures, such as the Performance-Oriented Mobility Assessment developed by Tinetti (41), BBS and TUGT (sensitivity 0.65–0.69 and specificity 0.51–0.66) (26) for identifying fallers with PD. Excellent sensitivity and specificity are required for a perfect prediction model. Our findings of a higher sensitivity (79%) than specificity (67%) suggest that the Mini-BESTest score has a higher accuracy among RF than N-RF. As recurrent falls lead to severe adverse physical and psychological effects (7, 27), identifying RF correctly (i.e. with higher sensitivity) is important so that timely fall prevention interventions can be given to potential recurrent fallers. Using the bootstrapping approach, we further calculate the 95% CI of the Mini-BESTest cut-off score, which lies between 16.9 and 21.0. A CI of 4.1 points is similar to the best minimal important change of the Mini-BESTest reported recently (42). The known CI could give us 95% confidence that this cut-off score is reliable and not due to measurement error. The optimal cut-off score and 95% CI is helpful to identify those who are at greater risk clinically for falling.
As the Mini-BESTest can be completed in 15 min, it is suitable for use as a screening tool to select high-risk patients for treatment. RF have particular deficits in their postural response, sensory orientation and anticipatory postural adjustment during baseline measures; therefore, treatment interventions that target these areas would be useful to prevent future falls in patients with PD. Strength training combined with sensory organization conditions increase the sensory organisation ability (43). Training with postural preparation and postural response to perturbation has been found to have a positive effect on balance performance (44, 45). Further study is needed to examine whether treatment interventions that enhance sensory integration, postural preparation and postural response abilities could reduce falls in RF.
This study has several limitations. First, the recruited patients were relatively mobile and community-dwelling; therefore, findings cannot be generalized to those who are severely impaired or institutionalized. Secondly, the use of multiple medications and high doses of levodopa is positively associated with a recurrent fall rate in patients with PD (10, 27). However, the use of medication was not analysed in our study. Thirdly, all of our baseline assessments were conducted during the “on medication” phase period; the test performance of subjects during the “off” phase is unknown and future studies need to compare the mobility levels of patients with PD in these two medication phases. Fourthly, we only included subjects who scored more than 20 in MMSE, and the relationship of cognition with recurrent falls in PD was thus not measured in our study.
To conclude, this is the first study to demonstrate that the Mini-BESTest score is an independent predictor of recurrent falls in individuals with PD. A cut-off score of 19 achieved a moderate sensitivity and specificity in predicting future recurrent falls in individuals with PD.
Acknowledgements
The study was supported by Hong Kong Parkinson’s Disease Foundation (5-ZH76). I acknowledge the assistance from NC Lau, YY Wong, X Shen for data collection.
References
