Berit Brurok, MSc1,2, Tom Tørhaug, MD1,3, Trine Karlsen, PhD2, Gunnar Leivseth, PhD, MD1,3,7, Jan Helgerud, PhD,5,6 and Jan Hoff, PhD1,4
From the 1St Olavs University Hospital, Department of Physical Medicine and Rehabilitation, Spinal Cord Injury Unit, 2KG Jebsen Center of Exercise in Medicine at Department of Circulation and Medical Imaging Norwegian University of Science and Technology, 3Norwegian University of Science and Technology, Faculty of Medicine, Department of Neuroscience, 4Norwegian University of Science and Technology, Faculty of Medicine, Department of Circulation and Imaging, Trondheim, 5Hokksund Medical Rehabilitation Centre, Hokksund, 6Telemark University College, Department of Sports and Outdoor Life Studies, Bø and 7Department of Clinical Medicine, Neuromuscular Diseases Research Group, University of Tromsø, Tromsø, Norway
OBJECTIVE: To compare peak oxygen uptake (VO2peak) between: (i) functional electrical stimulation lower extremity pulsed isometric muscle contractions combined with arm cycling (FES iso hybrid), (ii) functional electrical stimulation cycling combined with arm cycling (FES hybrid cycling), and (iii) arm cycling exercise (ACE) in individuals with spinal cord injury with level of injury above and below T6.
DESIGN: Cross-over repeated measures design.
Methods/participants: Individuals with spinal cord injury (n = 15) with level of injury between C4 and T12, were divided into groups; above (spinal cord injury – high, n = 8) and below (spinal cord injury – low, n = 7) T6 level. On separate days, VO2peak was compared between: (i) ACE, (ii) FES iso hybrid, and (iii) FES hybrid cycling.
RESULTS: In the SCI–high group, FES iso hybrid increased VO2peak (17.6 (standard deviation (SD) 5.0) to 23.6 (SD 3.6) ml/kg/min; p = 0.001) and ventilation (50.4 (SD 20.8) to 58.2 (SD 20.7) l/min; p = 0.034) more than ACE. Furthermore, FES hybrid cycling resulted in a 6.8 ml/kg/min higher VO2peak (p = 0.001) and an 11.0 litres/minute (p = 0.001) higher ventilation. ACE peak workload was 10.5 W (p = 0.001) higher during FES hybrid cycling compared with ACE. In the spinal cord injury – low group, no significant differences were found between the modalities.
CONCLUSION: VO2peak increased when ACE was combined with FES iso hybrid or FES hybrid cycling in persons with spinal cord injury above the T6 level. Portable FES may serve as a less resource-demanding alternative to stationary FES cycling, and may have important implications for exercise prescription for spinal cord injury.
Key words: electrical stimulation therapy; leg cycling; arm cycling; spinal cord injury; peak oxygen uptake.
J Rehabil Med 2013; 45: 00–00
Correspondence address: Berit Brurok, St Olavs Hospital, Department of Physical Medicine and Rehabilitation, Spinal Cord Injury Unit, Olav Kyrresgt 17, NO-7006 Trondheim, Norway. E-mail: berit.brurok@stolav.no
Accepted Sep 5, 2012; Epub ahead of print Jan 11, 2013
Introduction
Limited aerobic capacity in individuals with spinal cord injury (SCI) contributes to an increased cardiovascular risk profile (1). This limited aerobic capacity is associated with the level and extent of SCI. In particular, individuals with SCI at T6 level and above have reduced myocardial preload and myocardial contractility, resulting in reduced stroke volume and cardiac output (2). Individuals with SCI at or above T1 have a blunted heart rate response to exercise compared with able-bodied subjects, with maximal heart rates of approximately 130–140 bpm (3). Furthermore, in individuals with SCI at the T6 level and above, central innervation to the splanchnic vasculature is lacking. This, together with lack of an active muscle venous pump in the lower extremities, reduces venous return, blunts cardiac output, and as a consequence, blood pools in the lower extremities, and limits redistribution to the active muscles during exercise (3). To enhance a blunted cardiac output, and to increase peak oxygen uptake (VO2peak) functional electrical stimulation (FES) leg cycling combined with arm cycling (ACE) (FES hybrid cycling) is effective (4–7). Furthermore, FES hybrid cycling has a training effect on peak stroke volume when performed in aerobic high-intensity intervals (8). However, only a small number of individuals who may benefit take advantage of FES (9). This might be related to the fact that commercially available FES cycling modalities are expensive, require assistance and time for preparation, which limits their accessibility. Individuals with SCI regard exercise as an important aspect of quality of life, but report limited or no access to adapted training facilities (10).
At present there are several commercially available FES therapeutic exercise devices. Although the development of these has focused on accessibility and resource demands, further improvements and less resource-demanding modalities for improving aerobic exercise training quality in SCI individuals are needed. Using a portable FES modality for the lower extremities in conjunction with an individually chosen upper-body exercise mode might be such an alternative.
To our knowledge no previous study has compared VO2peak between portable FES lower extremity pulsed isometric muscle contractions combined with arm cycling (FES iso hybrid), FES hybrid cycling and ACE alone.
The primary aim of the present study was to compare VO2peak between FES-pulsed isometric lower extremity muscle contractions combined with arm cycling, FES cycling combined with arm cycling and arm cycling alone in individuals with SCI above and below the T6 level. The rationale behind dividing the participants at the T6 level is related to the different physiological response to exercise. Individuals with SCI above T6 (SCI–high) have a reduced sympathetic nervous system (SNS) outflow and supraspinal control to the splanchnic vasculature and lower extremity blood vessels, resulting in blood pooling during exercise (11). However, in individuals with SCI below T6 (SCI–low) the vasculature is generally adequately innervated, especially in the splanchnic vasculature, and clinical manifestations of a circulatory SNS dysfunction (i.e. venous pooling) during exercise may not be as marked (12, 13). We hypothesized that both FES iso hybrid and FES hybrid cycling would increase VO2peak more than arm cycling alone, and that there would be no difference in peak oxygen uptake between FES iso hybrid and FES hybrid cycling in individuals with SCI–high and SCI–low injuries.
Methods
Study design and population
A cross-over repeated measures design was used. A group of individuals with SCI (n = 15), without previous experience with FES training, were recruited from the Department of Spinal Cord Injuries at St Olavs Hospital, Trondheim University Hospital, Norway. Written informed consent was obtained from all individuals, and all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of the research.
The inclusion criteria were: having a traumatic SCI, at least 12 months since injury, spastic-paraplegia (lesion level between T1 and L1) and tetraplegia (lesion level between C4 and C8), with American Spinal Injury Association (ASIA) Impairment Scale (AIS) A (14). Participants had to be able to FES cycle for a minimum of 1 min. A pilot FES cycling test was performed prior to inclusion to test whether the participants fulfilled the inclusion criteria of being able to FES cycle for at least 1 min. Exclusion criteria were: current medical history of severe autonomic dysreflexia (systolic blood pressure > 30% from baseline, sweating/chills/piloerection/flushing, headache and heart rate decrease or for individuals with SCI above T1 an increase in heart rate), pacemaker and decubitus in any area. Participants were neurologically classified according to the International Standards of Neurological Classification of SCI (ISNSCI), skin health was assessed, and resting blood pressure and present cardiovascular disease was assessed in line with the recommendations from the American College of Sports Medicine (15). Body mass, in kg, was obtained using a SECA Digital Chair Scale 950 (SECA Hamburg, Germany), which was calibrated for seated weighing. The individual characteristics and baseline values of the participants are listed in Table I.
|
Table I. Individual and baseline characteristics of spinal cord injury (SCI)–high and –low groups |
||
|
SCI–high (n = 8) |
SCI–low (n = 7) |
|
|
TSI, years, mean (SD) |
12.9 (10.8) |
13.5 (11.7) |
|
Weight, kg, mean (SD) |
73.3 (8.4) |
74.9 (14.9) |
|
AIS Level of injuries |
A C4, C5, C7, C7, T2,T5, T5, T5 |
A T8, T9, T9, T10, T11, T11, T12 |
|
Men/women, n |
8/0 |
5/2 |
|
Age, years, mean (SD) |
35 (12.3) |
43.6 (12.8) |
|
Systolic RBP, mmHg, mean (SD) Diastolic RBP, mmHg, mean (SD) |
111 (19) 66 (10) |
121 (12) 71 (12) |
|
FES iso hybrid fatigue, min, mean (SD) FES hybrid cycling fatigue, min, mean (SD) |
3.4 (1.1) 3.4 (1.1) |
4 (1.1) 4 (1.1) |
|
HR rest, beats/min, mean (SD) |
75 (11) |
73 (10) |
|
TSI: time since injury; C: cervical level; T: thoracic level; RBP: resting blood pressure; SD: standard deviation; AIS: American Spinal Injury Association Impairment Scale; FES: functional electrical stimulation. |
||
Test protocols
The two FES hybrid VO2peak tests were performed in a random order after the peak ACE test. Randomization was accomplished by drawing a note from an envelope. The peak ACE test was performed at inclusion, before the two FES hybrid peak tests, to gain knowledge of the approximate time to start FES stimulation in order to avoid FES leg stimulation fatigue before arm cycling fatigue during the FES hybrid tests. The tests were performed on 3 separate days.
Two out of the 3 following criteria had to be met for a successful VO2peak test: respiratory exchange ratio (RER) ≥ 1.05, blood lactate level ([La–] b) ≥ 7 mmol and rating of perceived exertion (RPE) ≥ 15, BORG scale (6–20) (16). If these criteria were met, the mean of the highest VO2 within 3 consecutive 10-s measurements was used to calculate VO2peak.
Apparatus
Cardiopulmonary exercise testing was performed using the Metamax II Cortex ergospirometry system (Cortex Biophysik GmbH, Leipzig, Germany), through breath-by-breath measurements. Prior to all tests calibration of gas and volume were performed. For ACE, the ERGOMED 840L (Siemens, Oslo, Norway) was used.
For FES hybrid cycling the ERGYS II (Therapeutic Alliances Inc., Fairborn, OH, USA) and ERGOMED 840L were used. The wave shape parameters on the ERGYS II were set at 500 sine wave and 40 Hz pulsed. The maximum pulse intensity for each stimulation channel was set to the default 140 mA. The ERGYS II FES cycle ergometer attempted to maintain a pedalling cadence of 50 revolutions per min (rpm) by modulating the pulse amplitude intensity (mA) of the stimulation. The stimulation cut-off speed due to fatigue was set at 35 rpm. FES iso hybrid was conducted using the Motionstim 8 (Medel Electronics, Hamburg, Germany). FES was achieved by applying self-adhesive surface electrodes over the motor units of the quadriceps, hamstrings and gluteus muscles. Frequency was set at 40 Hz, pulse form was biphasic rectangular, and pulse width was 500 µs. A ramped protocol was used, where stimulation times were 3 s on and 2 s off.
In a capillary blood sample collected from a fingertip, haemolysed blood lactate concentration was measured by the portable Lactate Pro LT-1710 Analyzer (Arkray Factory Inc., KDK Corp., Kyoto, Japan). Lactate level was measured within 1 min after ending the VO2peak tests.
Heart rate (HR) was measured with a heart rate monitor (Polar Electro, Oy, Finland). Peak HR (HRpeak) was determined as the highest HR measured at the end of the VO2peak test. RPE (BORG scale 6–20) was recorded immediately after each test. Peak oxygen pulse (O2 pulse) was calculated as the ratio between the absolute VO2peak in ml and HRpeak.
Protocol for peak arm cycling exercise
Participants were seated in their manual wheelchair in front of the ACE. To secure a standardized position, the shoulder joint was horizontally aligned with the pedals and the elbows positioned slightly flexed at the point of furthest reach. To regulate sitting height and to achieve shoulder joint alignment with the ACE, 1 out of 3 different steel platforms was placed under the wheelchair. To enable performance, the tetraplegic individuals wore hand orthoses during ACE. Six min at 30 W of warm-ups was performed, directly, followed by peak testing on the ACE. During the test the participants were instructed to maintain a speed of 70 rpm. An individualized ramp protocol designed to reach VO2peak within 8–12 min was used. The protocol increased work rate in 5–10 W increments until the participants reached volitional fatigue. Typically 5 W increments were used for the tetraplegic participants and 10 W increments for the paraplegic participants. ACE peak power, in W, was determined by the highest power, in W, maintained for the last minute of the test. ACE protocols were identical during the two FES hybrid modes.
Protocol for peak functional electrical stimulation hybrid cycling
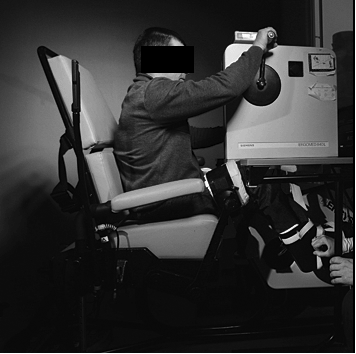
FES hybrid cycling was achieved by safely securing the arm ergometer on a table over the FES ergometer (Fig. 1). Participants were seated in the ERGYS II, where shoulder alignment to the ACE was achieved by elevating the ERGYS II. Electrode leads were connected to the electrodes, and stimulation thresholds were individually set (where the muscle starts to contract). After 6 minutes of ACE warm-up, the peak FES hybrid protocol was initiated together with ACE. For the participants with high FES fatigue, 2 min of FES warm-ups was started at a work-load corresponding to 5 min prior to peak ACE watt. This allowed the participants to reach fatigue at approximately the same time for both legs and arms. The 2 min of manually assisted FES cycling warm-up movements were conducted at an intensity of 50% of the individually set muscle thresholds. No resistance was provided on the flywheel during peak FES hybrid cycling. Individuals, who were able to, FES cycled throughout the whole test. Where necessary, the test personnel provided manual assistance to the pedalling to ensure that pedalling cadence did not drop below the stimulation cut-off speed of 35 rpm during the test.
Fig. 1. Functional electrical stimulation (FES) hybrid cycling and FES iso hybrid setup.
FES iso hybrid test
During FES iso hybrid testing the participants were seated in the ERGYS II (same set-up as during FES hybrid cycling). The self-adhesive electrodes were placed over the same muscles as during FES hybrid cycling. To achieve FES pulsed isometric muscle contractions, the ergometer pedals were set in the locked mode. Stimulus on the portable FES apparatus was individually set to achieve visible isometric muscle contractions. Due to high fatigue during FES in the participants in the present study, stimulation was started approximately in the 3 final min of the test to reach peak performance with simultaneous visible FES isometric muscle contractions. When necessary the stimulation intensity was increased by the test personnel to maintain visible contractions. The maximum stimulation intensity on the MotionStim 8 is 125 mA.
Statistical analyses
A Q-Q plot of VO2peak values did not reveal any indication for deviation from normal distribution of data. As the basis for applying FES during ACE is to facilitate venous return, comparisons of VO2peak between the test modalities was addressed separately for individuals with level of injury above and below T6 (SCI–high, n = 8) and (SCI–low, n = 7), respectively. With 2 groups (SCI–high and SCI–low) and 3 modalities (arm cycling, arm cycling combined with FES pulsed isometric contractions and arm cycling combined with FES cycling) we utilized a 2 by 3 analysis of variance (ANOVA) model for repeated measures. A p-value < 0.05 was considered statistically significant (two-tailed). To examine potential differences in VO2peak between type of exercise modality between SCI–high and SCI–low groups, an interaction term was included (full factorial model). Due to significant interaction, separate analyses for each group were carried out, using repeated measures ANOVA, pair-wise comparisons of peak oxygen uptake and related physiological parameters between the 3 test modalities, with adjusted nominal significance level set to 0.017 (Bonferroni method).
RESULTS
SCI–high group
In the SCI–high group, mean VO2peak was 6.0 ml/kg/min (95% confidence interval (CI): 2.9–9.0; p = 0.001) higher, mean peak ventilation (VE) was 8.2 l/min higher (95% CI: 0.6–14.9; p = 0.034) and O2 pulse was 2.6 ml/beat higher (95% CI: 1.2–3.9; p = 0.002) during FES iso hybrid compared with ACE. There were no difference in VO2peak and related physiological parameters between ACE, FES iso hybrid and FES hybrid cycling in the SCI high group (Table II).
FES hybrid cycling VO2peak and mean VE was 6.8 ml/kg/min (95% CI: 3.5–10.0; p = 0.001) and 11.0 l/min higher (95% CI: 7.7–14.4; p = 0.001), respectively, compared with ACE. FES hybrid cycling O2 pulse and ACE peak workload was 2.8 ml × beat–1 (95% CI: 1.3–4.2; p = 0.001) and 10.5 W (95% CI: 6.0–15.2; p = 0.001) higher compared with ACE. Blood lactate was 1.9 mmol/l (95% CI: 0.2–3.5; p = 0.029) higher compared with ACE, in the SCI–high group (Table II).
|
Table II. Peak effort testing in spinal cord injury–high group |
|||
|
Variables |
ACE n = 8 Mean (SD) |
FES iso hybrid n = 8 Mean (SD) |
FES hybrid cycling n = 8 Mean (SD) |
|
VO2peak |
|||
|
l/min |
1.24 (0.40) |
1.74 (0.40)* |
1.80 (0.40)** |
|
ml/kg/min |
17.6 (5.0) |
23.6 (3.6) * |
24.4 (4.1)** |
|
VE, l/min |
50.4 (20.8) |
58.2 (20.7) * |
61.4 (19.8)** |
|
RER |
1.14 (0.07) |
1.14 (0.07) |
1.18 (0.07) |
|
[La–]b, mmol/l |
7.5 (1.1) |
8.4 (1.9) |
9.4 (1.7)** |
|
HRpeak, beats/min |
149 (34) |
161 (21) |
163 (20) |
|
RPE |
18 (1) |
18 (1) |
18 (1) |
|
ACE, Wpeak |
72.5 (32) |
82.5 (27) |
83.0 (32)** |
|
O2 pulse, ml/beats |
8.2 (1.7) |
10.8 (1.7)* |
11.0 (2.0)** |
|
ACE: arm cycling; FES iso hybrid: pulsed isometric functional electrical stimulation combined with ACE; FES hybrid cycling: ACE combined with FES leg cycling; VO2peak: peak oxygen uptake; VE: ventilation; RER: respiratory exchange ratio; [La–]b: blood lactate concentration; HRpeak: peak heart rate; RPE: rating of perceived exertion. *Significant differences between ACE and FES iso hybrid (p ≤ 0.05); **significant difference between FES hybrid cycling (p ≤ 0.05) and ACE. |
|||
SCI–low group
VO2peak and related physiological parameters were not different between ACE, FES iso hybrid and FES hybrid cycling in the SCI–low group (Table III).
|
Table III. Peak effort testing in spinal cord injury–low group |
|||
|
Variables |
ACE n = 7 Mean (SD) |
FES iso hybrid n = 7 Mean (SD) |
FES hybrid cycling n = 7 Mean (SD) |
|
VO2peak |
|||
|
l/min |
1.74 (0.24) |
1.85 (0.32) |
1.89 (0.38) |
|
ml/kg/min |
23.7 (3.6) |
25.2 (4.6) |
25.6 (4.1) |
|
VE, l/min |
76.6 (13.4) |
82.0 (14.5) |
80.2 (21.1) |
|
RER |
1.25 (0.1) |
1.23 (0.1) |
1.23 (0.1) |
|
[La–]b, mmol/l |
9.3 (0.9) |
9.3 (1.8) |
10.6 (2.5) |
|
HR, beats/min |
185 (11) |
183 (10) |
182 (9) |
|
RPE |
19 (1) |
19 (1) |
19 (1) |
|
ACE, Wpeak |
96 (23) |
98 (21) |
98 (19) |
|
O2 pulse, ml/beat |
9.4 (0.9) |
10.1 (1.4) |
10.4 (1.8) |
|
ACE: arm cycling; FES iso hybrid: pulsed isometric functional electrical stimulation combined with ACE; FES hybrid cycling: ACE combined with FES leg cycling; VO2peak: peak oxygen uptake; RER: respiratory exchange ratio; VE: ventilation; [La–]b: blood lactate concentration; HR: heart rate; RPE: rating of perceived exertion. |
|||
Discussion
The main finding in the present study is that FES pulsed isometric lower extremity muscle contractions significantly augmented arm cycling peak oxygen uptake by 34% in the group with SCI level above T6. Furthermore, compared with ACE, FES hybrid cycling significantly increased VO2peak by 39% in the SCI–high group (Fig. 2), whereas no difference in VO2peak was found between the FES iso hybrid and the FES hybrid cycling modes. These findings indicate that both FES modalities may be equally effective in terms of improving aerobic capacity through activating the muscle-venous pump, increasing venous return and cardiac output in individuals with SCI–high. The rationale behind applying FES is to augment blood redistribution, reduce lower extremity blood pooling, increase cardiac pre-load and further improve arm aerobic capacity. Oxygen pulse, which is an indirect measure of stroke volume (the ratio between HRpeak and VO2peak), was significantly higher during FES iso hybrid and FES hybrid cycling compared with the ACE mode in the SCI–high group (2.6 and 2.8 ml/beat, respectively). The increase in oxygen pulse was lower compared with the increase in stroke volume found in the study by Hooker et al. (17). However, the current results provide evidence to support that FES hybrid cycling and FES iso hybrid increase stroke volume. The observation that ACE peak workload increased (10.5 W) with no change in HRpeak during FES hybrid cycling compared with ACE, supports the theory that there was an improved redistribution of blood from the legs to the arms. However, no significant difference was found in ACE peak power or HRpeak between ACE and FES iso hybrid. A likely explanation to the different findings may be the range of lesion levels and the different cardiovascular responses to exercise in the SCI–high group. Furthermore, the different method of applying FES, especially in terms of stimulation pattern; one being dynamic cycling movements and the other isometric muscle contractions, may partly explain the differences.
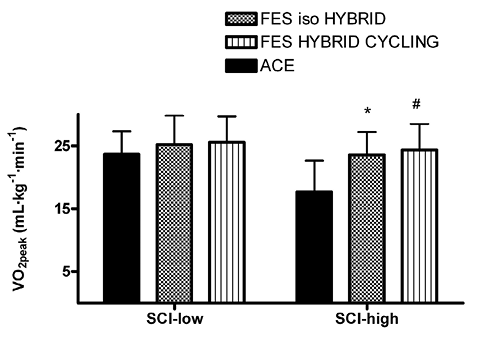
Fig. 2. VO2Peak measured for spinal cord injury–high and –low groups during arm cycling, arm cycling combined with functional electrical stimulation (FES) pulsed isometric contractions and arm cycling combined with FES leg cycling. Data are presented as mean (standard deviation; SD). ACE: arm cycling; FES iso hybrid: functional electrical pulsed isometric contractions combined with ACE; FES hybrid cycling: functional electrical cycling combined with ACE; VO2peak: peak oxygen uptake.
*Significant difference between ACE and FES iso hybrid; #significant difference between ACE and FES hybrid cycling. p ≤ 0.05.
As an interesting qualitative observation, two of the tetraplegic individuals with C5 and C6 SCI showed symptoms of hypotension (feeling faintness, nausea and pallor) during peak ACE testing, but not during the FES hybrid tests. The mechanism behind this was not assessed; however, it may be associated with the findings of Faghri et al. (18), where lower extremity FES during standing prevented orthostatic hypotension and augmented blood redistribution in tetraplegic individuals. Similarly, in a study by Ashley et al. (19), a group of tetraplegic individuals experienced immediate autonomic dysreflexia and a decrease in HR during lower-extremity FES. In the present study FES may also have masked hypotension by inducing autonomic dysreflexia. This effect remains to be determined, and is an important area of investigation. However, to propose the use of FES would be unethical if it induces autonomic dysreflexia, since it may induce fatal consequences, such as stroke, seizures and, in the worst case, death (20).
In contrast to our hypothesis, in the SCI–low group, no significant differences in VO2peak and related physiological parameters between the 3 tested modalities were found. This is also in contrast to other studies (21–24), where FES hybrid cycling VO2peak has been found to be significantly higher compared with ACE alone. A probable explanation for this may be methodological diversities in terms of lesion levels, determination criteria for VO2peak and individual FES training status. In previous studies, the individuals were exercise trained with FES, whereas in the present study, they were untrained and high fatigue during FES was common. High fatigue during FES may have affected the results in the present study, because the time-frame of FES delivery varied (some individuals received stimulation for 6 min and others for 3 min). Furthermore, in the SCI–low group, 5 of the participants needed to increase the FES current in order to achieve visible isometric contractions with the portable FES modality. Furthermore, during FES cycling, for the same participants, manual assistance was provided during the last minute, as opposed to two in the SCI–high group. The blunted cardiopulmonary response from applying FES in the SCI–low group may also be related to the fact that the splanchnic-vascular bed is innervated in individuals with SCI below T6, hence a limited, or no, haemodynamic response is triggered. Therefore, the effect from applying FES to the lower extremities during ACE may be associated with level of SCI, and not only FES training status per se. Since no acute physiological differences in peak oxygen consumption between the 3 tested modalities were found in the SCI–low group, future studies are needed to determine whether FES training affects the difference seen between individuals with SCI–high and SCI–low in the current study, as well as investigating the effect from training with the two FES hybrid modalities. To our knowledge, no study has assessed whether there exist a higher training effect from FES hybrid compared with ACE in a group with injury level below T6. As reviewed by Davis et al. (7) there are numerous positive effects from applying FES, i.e. increased cross-sectional area of muscle, shift in fibre type composition, improved leg blood circulation and positive psychosocial adaptations. Therefore, despite the lack of significant increase in acute VO2peak from applying FES during ACE, and the added expense of applying FES, it is still clinically worthwhile for individuals with SCI–low injuries.
From follow-up studies with able-bodied subjects it has been found that VO2peak is highly associated with morbidity and mortality (25, 26), indicating the importance of VO2peak as a prognostic factor and a measure of overall cardiovascular health. Therefore, to find effective exercise modalities in terms of training at the highest oxygen uptake is important. For the individuals in the SCI–high group in the present study, the two hybrid modalities may appear to result in the same level of oxygen uptake. Similar findings were made by Verellen et al. (4), where individuals with SCI between C7 and T12, demonstrated comparable values for VO2peak during FES hybrid cycling and FES rowing. An important perspective in the present study is that the FES iso hybrid modality, which is less expensive and more easily available, and which may be used in conjunction with a voluntary upper body exercise, seems to be equally effective as the stationary and more resource-demanding FES hybrid cycling modality. Considering the increased focus in recent years on exercise as a means of cardiovascular disease prevention in the able-bodied population, this should equally be a strong focus for individuals with SCI who are nearly twice as likely to develop cardiovascular disease (3, 27). A portable FES modality, in conjunction with, for example, outdoor hand-biking, wheelchair propulsion, cross-country skiing – sitting, is more easily accessible and may introduce an opportunity for a variability of exercises that may have the potential to improve training compliance, cardiovascular health and quality of life for individuals with SCI. This is an important area for future randomized controlled trials.
Study limitations
A low number of participants and lack of homogeneity in the groups reduce the statistical power and make it difficult to generalize the findings from the present study. The participants were not trained with FES, thus high fatigue during FES may affect redistribution of blood and reduce the effect on aerobic capacity. Furthermore, the SCI–low group included 4 individuals with level of injury between Th10 and Th12 who probably have some lower motor neurone injury to their legs. These individuals probably elicit weaker FES muscle contractions, with a concomitant lower metabolic increase and effect on the muscle venous pump, masking the effect from FES on VO2peak in the SCI–low group. To assess the mechanism behind the increased oxygen uptake from applying FES, blood pressure, peripheral arterial blood flow, and heart function could have been measured.
In conclusion, in individuals with a SCI above the T6 level, the addition of FES pulsed isometric contractions or FES cycling increased the measured peak oxygen uptake compared with arm cycling alone. However, no effect was found from applying FES in individuals with SCI below the T6 level. A portable FES pulsed isometric contraction device may serve as a more easily accessible and less resource-demanding alternative to stationary FES cycling. These findings may have important implications for exercise prescription for individuals with SCI.
AcknowledgementS
The authors would like to thank Professor Jon Magnussen at the Department of Public Health and General Practice, NTNU, for his critical reading of the manuscript. The work was supported by St Olavs Hospital – Trondheim University Hospital, Liaison Committee between the Central Norway Regional Health Authority and the Norwegian University of Science and Technology and St Olavs University Hospital, Department of Physical Medicine and Rehabilitation.
The authors declare no conflicts of interest.
References
