Anitra D. M. Koopman, MSc1, Maaike M. Eken, MSc1, Tijs van Bezeij, MD2, Linda J. M. Valent, PhD2,3 and Han Houdijk, PhD1,3
From the 1MOVE Research Institute Amsterdam, Faculty of Human Movement Sciences, VU University Amsterdam, Amsterdam and 2Heliomare Rehabilitation and 3Heliomare Research and Development, Wijk aan Zee, The Netherlands
OBJECTIVE: To investigate the cardiorespiratory strain experienced by patients over a day and during different types of rehabilitation therapies during a clinical rehabilitation programme. In addition, to investigate the use of the Borg scale as an instrument to monitor exercise intensity.
DESIGN: An observational, cross-sectional study.
SETTING: Rehabilitation centre in the Netherlands.
PARTICIPANTS: Eleven people after stroke (age range 20–71 years), 9 people with a lower limb amputation (age range 21–66 years) and 11 people with a spinal cord injury (age range 28–65 years). All participants were inpatients undergoing clinical rehabilitation.
Main outcome measures: Frequency distribution of percentage heart rate reserve (%HRR) and length of time heart rate (HR) > 40%HRR over one day, and mean %HRR, length of time HR > 40%HRR and HR > 70%HRR during different types of rehabilitation therapies were compared with the American College of Sports Medicine guidelines for achieving an aerobic training effect. The correlation coefficient between the Borg scale score and %HRR was assessed.
RESULTS: Patients’ mean HR was 114 min/day (standard deviation 92) > 40%HRR, of which 1 h was spent in therapy. In 5 out of 10 rehabilitation therapies (fitness, hydrotherapy, walking group, wheelchair group and cycling/handbike group) a mean HR > 40%HRR was reached and more than half of the time was spent > 40%HRR. A moderate correlation (R = 0.56) was found between Borg scale score and %HRR. All outcome measures showed large variation between and within patients.
CONCLUSION: In general, patients in a clinical rehabilitation programme experience adequate cardiorespiratory strain to potentially induce an aerobic training effect. The large variation in cardiorespiratory strain, however, necessitates individual monitoring to ensure proper exercise intensity. The Borg scale was shown to be of limited value for this monitoring, and therefore the use of HR monitors during rehabilitation should be considered.
Key words: cardiorespiratory strain; stroke; lower limb amputation; spinal cord injury; aerobic training effect.
J Rehabil Med 2012; 00: 00–00
Correspondence address: Han Houdijk, Heliomare R&D, Relweg 51, NL-1949 EC Wijk aan Zee, The Netherlands. E-mail: h.houdijk@heliomare.nl
Submitted February 19, 2012; accepted August 10, 2012
INTRODUCTION
Rehabilitation is the most common treatment modality for people after stroke and those with a lower limb amputation or spinal cord injury (SCI) to improve quality of life, in the absence of any curative therapy. The main objective of rehabilitation is to assist each patient in achieving the highest possible degree of individual physical and psychological performance (1). In many patients the capacity to perform and sustain activities of daily living (ADL) is limited (2–5), and is the result of reduced exercise capacity (3, 6, 7) and the elevated energy demands experienced in performing routine activities (8–13). In theory, regaining the ability to perform ADL could be achieved by lowering the energy requirements to perform ADL and/or by increasing the peak exercise capacity (5, 9, 11). The former can be achieved by task-specific training. For the latter, there is strong evidence that aerobic training can result in improvements in cardiorespiratory fitness and in functional gains (14–19). The American College of Sports Medicine (ACSM) guidelines for persons with chronic diseases and disabilities (20–23), including people after stroke, and those with a lower limb amputation and people with SCI, recommend that achieving an aerobic training effect requires exercising of large muscle groups at a frequency of 3–5 times a week, for a duration of 20–60 min (or multiple 10-min sessions), at an intensity of 40–70% heart rate reserve (HRR). To provoke an adaptation in cardiorespiratory fitness it is important to respect these guidelines and apply them in the rehabilitation programme.
It is unclear, however, whether the current clinical rehabilitation programme induces a sufficient amount of cardiorespiratory strain to provoke an aerobic training effect. Limited objective data exists. Janssen et al. (12) concluded that physical strain during a whole day in daily life of people with SCI is insufficient to induce an aerobic training effect. In addition, Janssen et al. (11) and Dallmeijer et al. (7) reported high levels of physical strain in people with spinal cord injury, but only during short periods of ADL tasks. However, these results are not obtained in inpatients during clinical rehabilitation. Only 3 studies were found that provide insight into the extent of cardiorespiratory strain induced during specific physical therapy treatment in a clinical rehabilitation programme in people after stroke (24–26). While Roth et al. (26) concluded that exercise intensity and duration were sufficient, both Mackay-Lyons & Makrides (25) and Kuys et al. (24) demonstrated that the intensity and duration of activities during physiotherapy was insufficient to result in a cardiorespiratory training effect in a group of people after stroke.
Lack of insight into the cardiorespiratory strain of clinical rehabilitation might partly be due to the fact that objective or subjective measures are not used routinely by therapists for determining exercise intensity during rehabilitation therapies. HR monitoring is an objective measure that is widely used to monitor exercise intensity in sports and leisure exercise. However, it is not commonly incorporated in rehabilitation practice. Alternatively, the Borg scale for perceived exertion offers an easy-to-use instrument that could easily be applied in a rehabilitation programme (27). Although it is a subjective measure, it has been reported to show a well-established relationship with heart rate in the able population (27). However, its use has been questioned for patients in rehabilitation (28).
Thus, based on the literature, no consensus exists on cardiorespiratory strain during clinical rehabilitation. Moreover, current literature have only described cardiorespiratory strain in people after stroke (and SCI) and for specific physical and occupational therapy sessions. Cardiorespiratory strain in other patient populations and during other rehabilitation activities is unknown. The main purpose of this study was therefore to investigate the cardiorespiratory strain in different patient populations over a whole day in a regular clinical rehabilitation programme, and to investigate the cardiorespiratory strain during specific rehabilitation therapies in which physical aspects are trained. These data were compared with the ACSM guidelines for aerobic training for persons with disabilities in order to assess the effectiveness of current clinical rehabilitation programmes for improving exercise capacity. A further purpose was to investigate the use of the Borg scale as a subjective measurement for use in rehabilitation therapies to regulate exercise intensity. These aims result in the following specific research questions:
• What is the cardiorespiratory strain, defined by %HRR, induced on a regular day during clinical rehabilitation, and for how much time does individual HR exceed 40% HRR?
• What is the mean cardiorespiratory strain, defined by %HRR, induced during specific rehabilitation therapies in which physical aspects are trained, and for how much time does individual HR exceed 40% and 70% HRR?
• What is the relationship between %HRR and the Borg scale score for the individual patient during rehabilitation therapies?
METHODS
Study design
An observational, cross-sectional study design was used to record the cardiorespiratory strain imposed during 3 days of clinical rehabilitation in patients after stroke and those with a lower limb amputation (LLA) or spinal cord injury (SCI). The intensity and duration of specific rehabilitation therapies, in which physical aspects are trained, were investigated by monitoring HR and perceived exertion.
Subjects
Study subjects were inpatients admitted in the clinical rehabilitation unit of Heliomare, Wijk aan Zee, The Netherlands. Inclusion criteria were: a diagnosis of stroke, LLA or SCI; and participation in several of the selected rehabilitation therapies that were expected to contribute to an increase in peak oxygen uptake (VO2peak) (Table I). In addition, subjects should be medically stable and able to perform a maximal incremental exercise test. The medical practitioner ascertained that potential participants would be able to perform this exercise test based on the absolute or relative contraindications for performing a maximal exercise test as proposed by the ACSM (29). To collect reliable data all participants should be able to understand and score the Borg scale, and, for proper use of the HR monitor, should have a body mass index < 30.0 and sufficient range of HRR > 55. In case of use of medications, the dose was kept constant during the study. The functional ability level for people after stroke was determined by using the Functional Ambulation Categories score (FAC-score), for people with a LLA K-levels were used, and for people with a SCI the Walking Index for Spinal Cord Injury scale (WISCI-scale) was used (30–32). This study was approved by the institutional review board. The general nature of the research was explained to the participants and all participants provided informed consent before inclusion in the study.
Protocol
Incremental exercise test. All participants performed a maximal incremental exercise test (IET) to establish the peak HR (HRpeak). People after stroke performed the IET on a recumbent bicycle (Lode Corival, Lode BV, Groningen, The Netherlands). The IET started with 2 min of unloaded pedalling and proceeded with an incremental load increase after each minute. Subjects diagnosed with a LLA or a SCI performed the IET on a hand bike, mounted on a Tacx (Tacx flow ergometer, Tacx, Wassenaar, The Netherlands). After each minute the resistance was manually increased. For all subjects, the test continued until they were no longer able to cycle or turn the pedals at 60–70 revolutions/min, or when they indicated wanting to stop. Starting load and increment size were selected such that the anticipated time to exhaustion was between 8 and 12 min. HR was measured using a Polar Heart Rate Monitor (Polar Heart rate Monitor, Polar Electro Oy, Kempele, Finland). VO2 and other cardiopulmonary exercise responses were obtained with the Oxycon Mobile (Oxycon Mobile, Viasys Healthcare, Hoechberg, Germany).
Assessment of cardiorespiratory strain during clinical rehabilitation
HR was monitored for 3 days (during 08.00–17.00 h) during which the subjects underwent their regular therapy programme. HR was recorded continuously at 5-s intervals using the heart rate monitor (3). Participants and therapist were blinded from these data during measurement, and the specific purpose of the measurement was not revealed to them in detail, in order to prevent a change in their normal behaviour or exercise intensity during the days of monitoring. Three days of monitoring were selected to obtain a more reliable estimate of daily strain. Furthermore, the ACSM guidelines recommend that exercise should occur on at least 3 days/week, which makes it necessary to observe whether this strain occurs on multiple days. Since we were also interested in the cardiorespiratory strain in selected therapies that are regarded as training physical aspects (Table I), the researchers selected the 3 days of monitoring in which these therapies were scheduled. Depending on the subject’s programme, these 3 days were either consecutive or non-consecutive. No attempt was made to influence the nature or duration of the therapies during this study. HR monitoring and the maximal IET were at most 2 weeks apart. Simultaneously with HR monitoring, the type and duration of activities performed by the subjects were registered by one of the investigators. In addition to monitoring strain and activities daily, 10 specific rehabilitation therapies and an ADL session were evaluated individually (Table I). At the end of these therapy sessions, the participants were asked by one of the researchers to score the intensity on the Borg scale.
|
Table I. Specified rehabilitation therapies |
|
Self-exercise programme (SEP) |
|
Sport |
|
Individual physical therapy (IPT) |
|
Individual occupational therapy (IOT) |
|
Fitness |
|
Hydrotherapy (HT) |
|
Walking group (WG) |
|
Wheelchair group (WCH) |
|
Special stroke programme (SSP) |
|
Cycling/hand bike group (HB) |
|
Activities of daily living (ADL) |
|
Specified rehabilitation therapies in which physical aspects were expected to be trained and in 3 of which the patients should be participating. |
Data analysis
HR data were processed using Matlab (Matlab, The Mathworks Inc., version R2007b, Natick, MA, USA). HRR is calculated as HRpeak minus HRrest. HRpeak was derived from the maximal IET. However, when during daily HR monitoring a higher heart rate was observed, this heart rate was considered as HRpeak. Resting heart rate (HRrest) was obtained from the lowest heart rate during the 3 monitoring days, usually occurring in the morning before getting out of bed or during an afternoon sleep. Using the Karvonen formula (33), the percentage of the HRR was calculated:
|
%HRR = |
HR–HRrest |
× 100 |
|
HRR |
The 3 days of data per subject were included in further analyses. To obtain cardiorespiratory strain, the recorded HR response data were categorized into 10% HRR intervals. The length of time the HR response of a subject remained in these intervals was determined and expressed in min/day. Thus, the cardiorespiratory strain could be obtained from a mean frequency distribution in the different HRR-zones (0–10%HRR, 10–20%HRR, …, 90–100%HRR) over all measured days of all subjects. In addition, the amount of time the HR was above 40%HRR was determined. For the specific rehabilitation therapies, the mean %HRR, and the amount of time the HR was above 40%HRR and above 70%HRR were calculated.
The normality of the variables was tested with a Kolmogorov-Smirnov test. Differences in HR responses over a whole day between the patients groups were determined by using a non-parametric Kruskal-Wallis test. For all other outcome measures, differences between the patient groups were determined with a parametric one-way analysis of variance (ANOVA) in SPSS version 19.0.
All scores given on the Borg scale during the specified rehabilitation therapies and corresponding mean %HRR were correlated per subject individually. To assess the relationship between these Borg scale scores and HR the Pearson correlation coefficient was computed. For all statistical tests the confidence level was set at 95% (p = 0.05).
RESULTS
This study included 11 subjects after stroke, 9 subjects with a lower limb amputation (LLA) and 11 subjects with SCI. Characteristics of all 31 participants are summarized in Table II.
|
Table II. Demographic and clinical characteristics of participants (n = 31) |
|||
|
Stroke patients (n = 11) CVA haemorrhagic (n = 1) CVA ischaemic (n = 10) |
LLA patients (n = 9) Transtibial (n = 4) Transfemoral (n = 3) Knee-exarticulation (n = 2) |
SCI patients (n = 11) Paraplegia (n = 8) Tetraplegia (n = 3) |
|
|
Age, years, mean (SD) [range] |
53.9 (16.2) [20.3–71.4] |
44.5 (15.8) [21.6–66.9] |
50.4 (13.4) [28.8–65.4] |
|
Sex M/F, n (%M) |
7/4 (63.6) |
8/1 (88.9) |
8/3 (72.7) |
|
Time in rehab, weeks, mean (SD) [range] |
5.0 (2.5) [1–9.7] |
11.7 (5.6) [2.3–18.7] |
9.9 (6.6) [2.9–26.7] |
|
Beta blocking medication, n (%) |
2 (18.2) |
0 (0) |
0 (0) |
|
Functional ability level |
1 × FAC 3 6 × FAC 4 3 × FAC5 1 × unknown |
2 × K2 1 × K3 5 × K4 1 × unknown |
5 × WISCI 0–6 2 × WISCI 7–13 2 × WISCI 14–20 2 × unknown |
|
HRpeak, beats/min mean (SD) [range] |
135.6 (16.8) [116–173] |
156.7 (21.4) [128–188] |
160.0 (11.6) [142–179] |
|
HRrest, beats/minute mean (SD) [range] |
60.1 (8.2) [49–78] |
61.1 12.0 [43–77] |
64.0 11.3 [47–86] |
|
% predicted HRmax# mean (SD) [range] |
79.4 (5.1) [73.1–89.3] |
88.3 (7.4) [77.7–99.3] |
92.6 (5.4) [85.9–103.2] |
|
VO2 peak, ml/min/kg, mean (SD) [range] |
19.0 (8.2) [7.5–39.3] |
23.4 (9.5) [10.2–35.9] |
21.4 (7.0) [13.9–31.4] |
|
RER, mean (SD) [range] |
1.19 (0.05) [1.12–1.25] |
1.16 (0.07) [1.07–1.23] |
1.20 (0.07) [1.10–1.31] |
|
Time in rehab: Time in rehabilitation before data collection. # HRpredicted max = 208–0.7 × age (45). n: number of subjects; CVA: cerebrovascular accident; LLA: lower limb amputation; SCI: spinal cord injury; M: male; F: female; RER: respiratory exchange ratio. |
|||
Heart rate responses
The mean HR recording time was 7 h 50 min/day (standard deviation (SD) 55). Fig. 1 shows the mean distribution of the cardiorespiratory strain over a day for the total group and 3 patient subgroups. The peak of this distribution for the total group occurred in the zone of 20–30%HRR (mode = 26%HRR, median = 29%HRR). Patients with a LLA and SCI showed a peak in the 20–30%HRR-zone (LLA: mode = 26%HRR, median = 29%HRR; SCI: mode = 24%HRR, median = 28%HRR). In the group of patients after stroke the cardiorespiratory strain was distributed more evenly over the HRR zones between 10–40%HRR, but with similar mode and median strain compared with both other groups (stroke: mode = 29%HRR, median = 29%HRR).
Fig. 1. Mean length of time per day (in minutes) that the heart rate is within particular zones of the heart rate reserve (HRR) (with standard deviation) for the total group and the different patient groups. n = number of patients.
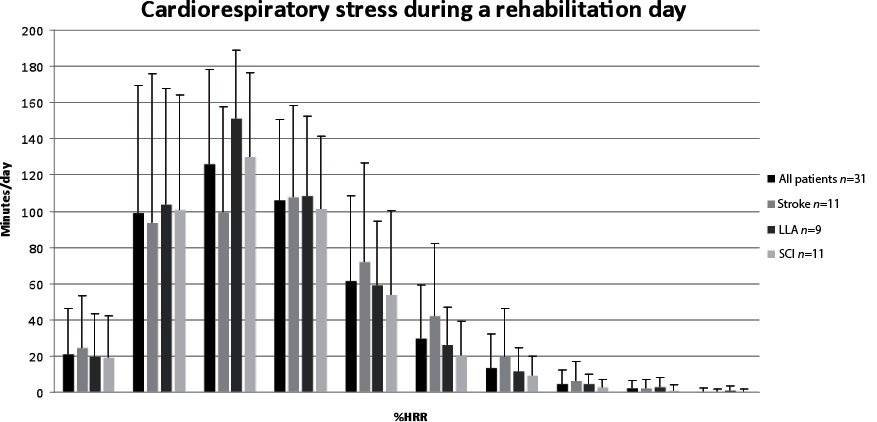
A mean of approximately 2 h (114 min (SD 92)) per day were spent above 40%HRR (Fig. 2). A large variation was noticed. One subject spent less than 5 min above 40%HRR and 3 subjects spent more than 3 h/day above 40%HRR. During the 3 days monitored, 23 subjects (75%) reached a HR above 40%HRR for more than 20 min each day in consecutive bouts of > 10 min. Subjects spent a mean of 120 min (SD 56) in therapy and 337 min (SD 80) in non-therapy activities. In nearly half of the time spent in therapy (45% (SD 28%), equal to 53 min (SD 39)) the HR was above 40%HRR. During non-therapy time the HR was above 40% HRR for 17% (SD 19%) of the time, equal to 67 (SD 74) min. The relative contribution of therapy to each %HRR zone increased with increasing %HRR.
Fig. 2. Box-plot representation of the length of time per day (in minutes) that the heart rate is above 40% heart rate reserve (HRR) for the total group and the different patient groups. n = number of subjects; Q1 = splits lowest 25% of the data; Q3 = splits highest 25% of the data.
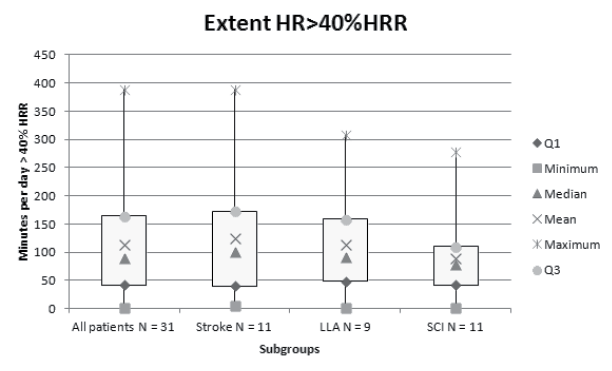
Table III shows the cardiorespiratory strain induced by various rehabilitation therapies investigated in this study. The cycling/hand bike group for people with a LLA and a SCI shows the highest mean %HRR and the highest percentages time HR was above 40% and 70% HRR (Table III). The following rehabilitation therapies also elicited a high mean %HRR and stressed the cardiorespiratory system above 40%HRR for at least half of the therapy duration: fitness, hydrotherapy, walking group for subjects after stroke and subjects with SCI and the wheelchair group for subjects with a LLA or SCI. Again, large variation was observed between subjects.
|
Table III. Cardiorespiratory strain induced by various rehabilitation therapies and activities of daily living |
|||||||||||
|
Rehabilitation therapy |
SEP |
Sport |
IPT |
IOT |
Fitness |
Stroke/ SCI – HT |
Stroke/ SCI – WG |
SSP |
LLA/ SCI – WCH |
LLA/ SCI – HB |
ADL |
|
Total, n |
29 |
68 |
83 |
53 |
29 |
10 |
15 |
17 |
6 |
17 |
31 |
|
%HRR, mean (SD) |
40 (15) |
35 (14) |
36 (14) |
32 (15) |
42 (12) |
45 (14) |
47 (16) |
40 (10) |
49 (18) |
55 (13) |
33 (17) |
|
Mean % of Time HR > 40%HRR, %, mean (SD) |
47 (37) |
37 (35) |
43 (35) |
25 (32) |
54 (39) |
59 (37) |
60 (41) |
43 (31) |
59 (44) |
77 (20) |
34 (35) |
|
Mean % of Time HR > 70%HRR, %, mean (SD) |
13 (21) |
7 (18) |
7 (16) |
7 (21) |
13 (18) |
18 (33) |
33 (38) |
10 (16) |
27 (33) |
39 (31) |
8 (21) |
|
Duration, min, mean (SD) |
25 (8) |
28 (5) |
26 (5) |
30 (14) |
46 (15) |
30 (14) |
22 (4) |
36 (13) |
23 (5) |
51 (21) |
10 (8) |
|
Therapies that exceeded the selected threshold for sufficient cardiorespiratory strain (a mean HR of > 40% HRR) are printed in bold. SEP: self-exercise programme; IPT: individual physical therapy; IOT: individual occupational therapy; Stroke/SCI – HT: hydrotherapy; Stroke/SCI – WG: walking group; SSP: Special Stroke Program; LLA/SCI – WCH: wheelchair group; AMP/SCI – HB: cycling/hand bike group; ADL: washing, clothing and self care. |
|||||||||||
Borg scale
On average, a moderate correlation (R = 0.56) was found between the score given on the Borg scale and the corresponding %HRR of the selected rehabilitation therapies during 3 days of monitoring. Ten out of 31 subjects (32%) showed a strong correlation (0.7 < R < 0.85). Eleven subjects (35%) showed a moderate correlation (0.5 < R < 0.7) and 10 subjects (32%) showed a weak correlation (R < 0.5) (34). People after stroke showed the weakest correlation (R = 0.46 ± 0.23) compared with people with a LLA and SCI (R = 0.63 ± 0.20; R = 0.59 ± 0.25), but no significant difference was found between the groups.
DISCUSSION
The ACSM guidelines recommend exercise of large muscle groups at a frequency of 3–5 times a week for a duration of 20–60 min (or multiple 10-min sessions) at an intensity of 40–70%HRR to achieve an aerobic training effect (20–23). According to these guidelines, our results showed that during clinical rehabilitation, people after stroke, those with a lower limb amputation and those with a SCI experience cardiorespiratory strain of such intensities that an aerobic training effect can be expected. In 75% of subjects participating in our study the HR was above 40%HRR for more than 20 min/day on all 3 days that they were monitored. The HR of the monitored subjects was above 40%HRR for a mean of 2 h (114 min) per day, which is well above the ACSM guidelines. However, the results are mean group data with considerable outliers, and a large variation existed among the subjects. This might be due to the differences in clinical characteristics of the participants and their treatment goals in our heterogeneous sample. Nevertheless, despite this large variation, most subjects did meet the standards for provoking an aerobic training effect.
Of the 2 h per day that on average subjects’ heart rate was above 40%HRR, nearly half of the time was recognized as time in therapies in which physical aspects were trained. Moreover, the relative contribution of therapy to each %HRR zone increased with increasing %HRR, indicating that therapy becomes more important in eliciting higher levels of cardiorespiratory strain in patients. The remaining hour of the time that the heart rate was above 40%HRR was ADL and free time, called non-therapy time. Part of the time the HR was above 40%HRR during non-therapy time might have been due to an after-effect of an intensive rehabilitation session and occur as recovery time when the rehabilitation session itself had already ceased. An additional reason for high cardiorespiratory strain in non-therapy time has been suggested to be elevated energy demands (HR > 40%HRR) to perform ADL, including washing, clothing and self-care (35). However, our results did not show high levels of %HRR during ADL and similar activities trained during occupational therapy (mean 33%HRR and 32%HRR, respectively). In addition, the duration of periods of ADL with a HR>40%HRR were too short (10 min (SD 8)) to induce a cardiorespiratory training effect. This is in accordance with similar research (36, 37), which found that most of the time not spent in therapy was at a low aerobic level. It can hence be concluded that therapies targeting physical factors are important and effective for inducing cardiorespiratory strain during a day of clinical rehabilitation. Nevertheless, although part of the time above 40%HRR occurs in non-therapy, and might be induced by recovery from therapies, it cannot be ruled out that non-therapy time also contributes to an aerobic training effect.
Our results showed that 5 out of 10 rehabilitation therapies analysed in this study induced a mean HR above 40%HRR (Table I). The handbike group especially proved useful for improving physical fitness in people in clinical rehabilitation, which is in accordance with Dallmeijer et al. (38). This is in contrast, however, to previous research, which found that cardiorespiratory strain induced during physical therapy sessions in patients after stroke was insufficient to result in a cardiorespiratory training effect (24, 25, 39). The lack of agreement between findings of previous studies and the results from the present study can be explained by several factors. The first factor might be different inclusion criteria. We only included stroke patients who were able to walk, whereas Kuys et al. (25) included both walkers and non-walkers (24). As a consequence, it might not be surprising that they found a lower %HRR during physical therapy (24%HRR), because non-walkers did not participate in activities that we showed could improve cardiorespiratory fitness. A second important explanation is the determination of HRpeak. Previous studies obtained HRpeak using the formula HRpredicted max=220–age (40) (or adjusted formula for patients taking beta-blocking medication: HR = 85%[220–age]) (41)). In contrast, subjects participating in the present study performed a maximal IET for determining the HRpeak. Because of the relatively large prediction errors of the above-mentioned formulae, we considered this to be the most accurate method (42). It should be noted that this method relies on the motivation and capacity of participants to achieve a maximal performance. When the maximal capacity is not reached in this test, HRpeak will be underestimated and average load in terms of %HRR will be overestimated. However, the majority of the subjects assessed in this study (24 out of 31) did meet the criteria of a maximal IET (meeting a minimum of 2 of the 3 following criteria: breathing frequency above 35; respiratory exchange ratio (RER) above 1.1; and a plateau in VO2 with increasing exercise intensity (< 150 ml) (43, 44)). This is further supported by the fact that, on average, most subjects reached HRpeak close to their predicted maximal heart rate, although the group of patients after stroke did achieve somewhat lower values in comparison with the 2 other patient groups (Table II). For a small number of subjects we had doubts that peak aerobic performance was approached during the incremental test. These participants were the outliers in Fig. 2, and hence the underestimation of their maximal HR can explain these outliers. Nevertheless, we can consider that in most subjects the observed HRpeak represents the real maximal HR and no overestimation of the induced cardiorespiratory strain is made. The use of an IET to obtain HRpeak can be considered as a major strength of this study.
Based on our results we can conclude that, in theory, cardiorespiratory strain is sufficient to expect an aerobic training effect during rehabilitation. However, it should be realized that we did not evaluate the outcome of clinical rehabilitation regarding aerobic fitness. In our conclusion we assume that the lower boundary of the ACSM recommendations will be sufficient to obtain an effect in this group of relatively people with physical activity levels. However, different thresholds for obtaining a training effect have been proposed by other studies (11, 12, 7). Moreover, a recent study of Baert et al (39) did not find a systematic change in aerobic capacity in a group of stroke patients during regular rehabilitation. However, the cardiorespiratory strain of these patients during their rehabilitation was not assessed. Thus, it is not known whether the strain in their programme complied with our results and the ACSM recommendations. The actual effect of the observed cardiorespiratory strain in this study thus remains to be established.
The large within- and between-subject variation in our data suggests that while, on average, patients are sufficiently stressed during rehabilitation, individual monitoring is essential for providing the right load to the right patient. The Borg scale provides an easy way of assessing exercise load (28). Our results indicated, however, that the Borg scale is not a valid instrument to regulate aerobic exercise intensity during the different rehabilitation therapies in the majority of patients. The mean correlation between %HRR and Borg scale is low, and only a few participants showed a correlation of sufficient strength to allow the clinician to rely on this outcome. This negative finding is in agreement with previous observations (45). This challenges the use of the Borg scale as a valid measure of aerobic exercise intensity in people with disabilities, and motivates the use of HR monitoring during rehabilitation therapies to assess and regulate exercise intensity.
Limitations
The sample of participants in this study was heterogeneous in terms of diagnoses and severity of impairments, which probably caused the large variation in outcome measures. However, we believe that it was important to capture the existing variability of the patients in rehabilitation. Although this study allows us to make a general statement about the cardiorespiratory strain in clinical rehabilitation, which seems to comply with minimal requirements in the majority of the patients, it should be realized that this differs substantially among individual patients. Secondly, our data was captured within a single rehabilitation centre in the Netherlands. The generalization of our results to other settings should therefore be treated with some care. van Langeveld et al. (46), however, observed similarities among Dutch rehabilitation in therapy content and therapy time. Therefore, our data presents a scarce objective indication of the cardiorespiratory strain induced during clinical rehabilitation, and might be used as a bench-mark for other settings.
Clinical messages
• During clinical rehabilitation cardiorespiratory strain is adequate to provoke an aerobic training effect.
• Large variation in daily cardiorespiratory strain between patients was observed, hence individual monitoring is advised.
• The Borg scale is not adequate to monitor exercise intensity during clinical rehabilitation; the use of HR monitors should be considered.
ACKNOWLEDGEMENTS
The authors are grateful to their participants for the involvement in this study. We would also like to acknowledge the support of Roos Kok, Ruth Sijsma and Richard Fickert for their clinical assistance and advice, and Geeske Dijkshoorn, Astrid Haijtink and Jetty Roos for their assistance in data collection.
References
