Jaana Sarajuuri, LicPsych1, Matti Pasanen, MSc2, Marjo Rinne, PhD2, Matti Vartiainen, MSc2, Tommi Lehto, PT1 and Hannu Alaranta, MD, PhD1†
From the 1Käpylä Rehabilitation Centre, Helsinki and the 2UKK Institute for Health Promotion Research, Tampere, Finland
OBJECTIVE: To explore the relationship between cognitive and motor performance in physically well-recovered men with traumatic brain injury.
DESIGN: Cross-sectional explorative study in a national neurorehabilitation centre.
SUBJECTS: Men with post-acute traumatic brain injury (n = 34; aged 19–55 years) who had recovered well physically.
METHODS: Cognitive performance (attention, information processing, cognitive flexibility, motor regulation, praxis of the upper limbs) and motor performance (postural balance, agility, rhythm-co-ordination) were assessed. Partial rank correlation coefficients and analyses of covariance were used to assess the associations between these tests.
RESULTS: Associations were found between the time taken in both Trail Making tests and performance time in the agility test (r = 0.57). The score on the Digit Symbol test correlated with time in the agility test (r = –0.52). Patients with normal performance in verbal fluency performed the tests of dynamic balance and agility 26% more quickly than those with abnormal performance. Moreover, patients with normal performance in the reproduction of rhythmic structures were 20% faster in the dynamic balance test. Motor functions of the hands associated with all the motor-performance test results.
CONCLUSION: Measures of information processing, attention and executive functioning may be associated with motor performance. Apart from the theoretical relevance, the finding of an association between cognitive and motor performance may have clinical relevance with regard to rehabilitation.
Key words: brain injuries; motor skills; neurobehavioral manifestations; rehabilitation; neuropsychology.
J Rehabil Med 2012; 00: 00–00
Correspondence address: Jaana Sarajuuri, Käpylä Rehabilitation Centre, Nordenskiöldinkatu 18 B, PO Box 103, FIN-00251 Helsinki, Finland. E-mail: jaana.sarajuuri@invalidiliitto.fi
Submitted November 3, 2011; accepted July 11, 2012
INTRODUCTION
The consequences of traumatic brain injury (TBI) influence human functions along a continuum ranging from the altered physiological functioning of cells through neurological and psychological impairments to medical problems and disabilities. All these affect the person concerned, as well as his or her family, friends and community, and society in general. The primary and secondary effects of TBI often result in combined physical and neuropsychological sequelae that seriously challenge the afflicted person’s autonomy.
The mechanics of the most common form of TBI, acceleration–deceleration injuries, often cause lesions in the orbital and lateral surfaces of the frontal and temporal lobes (1). The neurocircuitry and structures of these areas are subservient to attention, memory and executive functions, which are commonly disrupted following TBI. Acceleration–deceleration forces may also have shearing effects on the long nerve fibres coursing throughout the brain. This type of injury, known as diffuse axonal injury, commonly manifests in the medial frontal lobes, the corpus callosum, and the superior cerebellar peduncles (1). It is now known that diffuse TBI evokes complex cellular and subcellular responses in both the neuronal somata and its axonal branches (2). Many patients with TBI, especially those with brainstem injuries, show fatigue and generalized slowing of information processing even if the injury is mild (3, 4). Cognitive abilities also influence and are influenced by emotional and behavioural difficulties, as well as diverse physical problems (5). It is well established that even minor changes in the ability to attend, process, recall and act upon information can profoundly affect an individual’s daily functioning (5).
Common consequences for motor performance following TBI include postural instability and motor-co-ordination deficits, which may persist for years even in patients with no obvious neurological deficits (6–11). Balance problems are most evident in deep parenchymal brain damage or focal cerebral lesions (7). According to McFadyen et al. (12), residual effects on walking remain even though locomotor capacity is maintained in a highly functional person with TBI. In addition, studies have shown that a substantial proportion of young patients with TBI who can walk independently are unable to run (13, 14). Rinne et al. (6) further found that physically well-recovered men with TBI had impaired balance and agility compared with healthy men, and in a rhythm co-ordination test they had difficulties in starting and sustaining simultaneous rhythmic movements of the hands and feet.
Motor performance and cognition have usually been studied in isolation. However, in recent years there has been increasing evidence that neural regions typically associated with cognitive performance may also be recruited during the performance of motor tasks (15–17). According to Sosnoff et al. (17), there is a dearth of research examining the association between deficits in motor and cognitive functions. The evaluation of the relationship between cognitive and motor performance in patients with TBI has accordingly been very limited. Investigations of balance in association with other symptomatic and psychometric assessments have shown that persons with TBI often demonstrate increased reliance on visual input, and tend to sway more than normal control subjects (7, 13). The findings of Geurts et al. (8) indicate a possible association between balance and cognitive performance after mild TBI. Furthermore, having examined the relationship between balance, attention and dual-task performance in individuals with acquired brain injury, McCulloch et al. (16) observed dual-task costs with variable patterns across subjects: motor slowing, reduced cognitive accuracy, and decrements in both tasks. Parker et al. (18) studied subjects who had sustained concussion and healthy subjects, and found that both groups had slower walking speed during dual-task conditions compared with routine walking, but the subjects with concussion were more markedly affected. It has also been found that dual-task behaviour is deficient in people with moderate to severe TBI, even those with a high level of locomotor performance (19, 20). Measures of executive functioning and attention may also be associated with locomotor behaviour in complex environments following TBI (19).
Apart from the theoretical relevance of a possible association between cognitive and motor performance, this finding may also have potential clinical relevance with regard to rehabilitation. Combining therapeutic cognitive and motor activities may approximate the demands of everyday life more closely than artificially separating them in isolated therapy sessions. If rehabilitation is to be successful, therefore, it is essential to address the problems from multiple perspectives and to foster comprehensive and trans-disciplinary teamwork (5, 21). According to Ponsdorf et al. (22), the rehabilitation process needs to be person-focused rather than discipline-focused. This is considered essential to the success of rehabilitation following TBI, especially because people with TBI have such difficulty in generalizing what is learned in one setting to another.
The objective of this study was to explore the relationship between cognitive and motor performance in terms of postural balance, agility and gross motor rhythm co-ordination in men with TBI whose physical recovery was good. This is an explorative study with no predefined hypotheses, and one of its aims was to provide additional guidance for further research on the relationship between cognitive and motor performance.
METHODS
Participants
Men with a primary diagnosis of TBI consecutively attending a national rehabilitation centre (Käpylä Rehabilitation Centre, Helsinki, Finland) and who fulfilled the criteria for inclusion in the study were recruited over one year. Eligibility for the study in terms of the type and time of the injury was verified from medical files. A total of 41 patients with TBI were interviewed on the first day of their rehabilitation period in the centre to ensure their suitability for the study. The inclusion criteria were: (i) age 19–55 years; (ii) body mass index (BMI) less than 35; (iii) normal Mini Mental State Examination (MMSE; normal > 24/30), which is a widely used method for screening mental status in adults, testing orientation, attention, immediate and short-term recall, language, and the ability to follow simple verbal and written commands (23); (iv) ability to maintain initial test positions; (v) ability to perform a 2 km Walk Test developed at the UKK Institute (24); and (vi) ability to run a short distance, which was also the criterion for the patients to be considered physically well-recovered. A further requirement was that the patients were more than 1 year post-injury. Of the 41 patients with TBI, 2 refused to participate in the study and 5 were ineligible due to the inclusion criteria. In total, 34 men with TBI (mean age 34 years) met the criteria. All the patients gave their informed consent. Demographic and injury-related information is shown in Table I. The study was approved by the Ethics Committee of Ophthalmology, Otorhinolaryngology, Neurology and Neurosurgery of the Helsinki and Uusimaa Hospital District, Finland.
|
Table I. Demographic and injury-related characteristics |
|
|
Characteristics |
TBI group (n = 34) |
|
Age, years, mean (SD) |
35 (10) |
|
Height, cm, mean (SD) |
177 (7) |
|
Body mass, kg, mean (SD) |
80 (15) |
|
Body mass index, kg/m2, mean (SD) |
25.5 (3.9) |
|
Length of education, years, mean (SD, range) |
11.3 (1.4, 8–12) |
|
Time from injury, months, median (range) |
24 (12–144) |
|
Mechanism of injury, frequency (%) |
|
|
Motor vehicle collision |
18 (53) |
|
Falling |
7 (21) |
|
Pedestrian-auto collision |
4 (12) |
|
Assault |
4 (12) |
|
Bicycle collision |
1 (3) |
|
Glasgow Coma Scale scorea |
|
|
Mild (13–15) |
10 (29) |
|
Moderate (9–12) |
1 (3) |
|
Severe (3–8) |
15 (44) |
|
Post-traumatic amnesia, frequency (%)b |
|
|
Mild (< 24 h) |
1 (3) |
|
Moderate (1–7 days) |
7 (21) |
|
Severe (> 7 days) |
10 (30) |
|
Very severe (> 4 weeks) |
15 (46) |
|
Brain CT/MRI findings, frequency (%) |
|
|
Contusion and/or intracranial haematoma |
26 (77) |
|
Diffuse axonal injury |
5 (15) |
|
Signs of severe intracranial pressure |
4 (12) |
|
Neurosurgical treatment, frequency (%) |
|
|
Craniotomy |
2 (6) |
|
Type of rehabilitation after injury, frequency (%) |
|
|
Outpatient |
|
|
Neuropsychological rehabilitation |
24 (71) |
|
Physical therapy |
14 (41) |
|
Speech therapy |
3 (9) |
|
Occupational therapy |
4 (12) |
|
Inpatient rehabilitation |
6 (18) |
|
Medical treatment for sleeping, mood problems or |
|
|
pain, frequency (%) |
19 (56) |
|
aGCS scores were registered at acute hospital phase in 26 patients’ medical files; registration was missing in 8 patients’ files. b1 value is missing. TBI: traumatic brain injury; SD: standard deviation; CT: computed tomography; MRI: magnetic resonance imaging. |
|
Procedures
Computed tomography (CT) and magnetic resonance imaging (MRI) scans, information concerning Glasgow Coma Scale scores, neurosurgical interventions and the length of post- traumatic amnesia were evaluated from medical files by a neurologist. In addition, a board-certified clinical neuropsychologist verified the previous neuropsychological sequelae of the patients from medical files. Various combinations of problems were identified, including: (i) a tendency to become fatigued; (ii) slowness of information processing; (iii) disorders in attention and concentration; (iv) disorders in learning and memory; (v) disturbances in executive skills, such as initiation, planning and self-monitoring, or in judgement; (vi) difficulties in modulating affective states, including irritability and emotional lability; and (vii) disorders in language communication, such as tangentiality, hyperverbality and ineffective word retrieval. The cognitive examination was conducted after the recruited patients had given their informed consent on the first day of their rehabilitation period.
Motor performance was evaluated on 5 tests measuring balance, agility and rhythm co-ordination. Before starting, the tester demonstrated the performance of each test and the patients were allowed to practise it once. Two experienced physiotherapists administered the tests.
Measures
Neuropsychometric testing. On the basis of the results of the very few studies examining the relationship between cognitive and motor performance in patients with TBI, neuropsychological measures of information processing, attention and executive functions were assumed to show a positive relationship with motor acts in terms of speed and fluency, particularly when attention is divided and the regulation of voluntary movements is required (8, 16, 18–20). Consequently, a cognitive screening battery from the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) (25, 26) and several complementary standardized neuropsychological measures were used to evaluate patients’ cognitive functioning. The CERAD battery consists of tests of verbal fluency (naming animals), the modified 15-item Boston Naming Test, word-list learning, recall and recognition, constructional praxis and its recall, and the MMSE. The range of the MMSE was restricted because it was also used as an inclusion criterion. Trail Making tests (TMT) (27) and the Digit Symbol subtest from the revised Wechsler Adult Intelligence Scale (WAIS-R) (27, 28) were used to assess attention, complex information processing, visual conceptual understanding, visuomotor tracking and cognitive flexibility.
The motor functions subtests (11 tasks) and acoustico-motor organization subtests (3 tasks) from Luria’s Neuropsychological Investigation (LNI) (27, 29) were administered in order to assess the motor regulation and praxis of the upper limbs. Investigation of motor functions, according to the LNI, involves an analysis of praxis, i.e. the complex forms of construction of voluntary movements. The regulation of motor acts relates to executive functions and their role in producing voluntary movements. The motor-function subtests used in this study comprised 4 tasks of simple forms of praxis (separating and bringing together the fingers of both hands, reproducing two positions of the hand shown by the examiner, reproducing particular positions of the hands demonstrated by the examiner while sitting facing the subject, and reproducing the positions of the hands of the examiner while sitting facing the patient and touching the ipsilateral or contralateral ear and eye [Head’s test]); 2 tasks of complex forms of praxis (carrying out an action with objects that are not present including showing how to pour and stir tea, thread a needle, and cut with scissors, performing symbolic actions, threatening [shaking a fist] and waving goodbye); 3 tasks of dynamic organization (placing both hands in front, one with the fist clenched and the other with the fingers outstretched, and then simultaneously changing the positions of both hands [Ozeretskii-test], placing the hands successively in 3 different positions (making a fist, extending the fingers with the palm vertical and resting flat on a table), drawing a design comprising twoalternating components; and 2 tasks requiring the speech regulation of motor acts (knocking twice when the examiner knocks once and vice versa, and showing a finger when the examiner shows his fist and vice versa). The acoustico-motor-organization subtests comprised two tasks focused on the perception of rhythmic structures (counting the number of taps included in single groups of 2 or 3 rhythmic taps, and counting the number of taps included in series of groups), and one task requiring the reproduction of rhythmic structures (reproducing rhythms from a pattern presented acoustically).
The results of the verbal-fluency, word-list recall and constructional praxis (copying) tasks were dichotomized into the categories “normal” and “pathological” in accordance with the cut off-scores of the CERAD test. A similar categorization was used for motor functions of the hands, the speech regulation of motor acts, and the perception and reproduction of rhythmic structures. In line with the LNI, the performance was categorized as “pathological” even if only 1 of the tasks of a certain function was failed (27, 29). To be considered “normal” the participant had to perform the tasks without any difficulty. Given that the scoring in the reproduction of rhythmic structures test is based on the number of faultless trials in proportion to the maximum number of trials, the following dichotomous variable was formed: “normal” 6–7 points, “pathological” 0–5 points.
Motor performance. Indications of static and dynamic postural instability, retarded velocity and difficulties in motor co-ordination have been reported in TBI patients with good motor recovery, leading to recommendations in the literature to assess balance, gait, co-ordination, rapid alternating movements and proprioception among well-recovered patients (9, 11, 12). The motor tests chosen have proved to be reliable for assessing mild physical impairment after TBI (30). From the clinical perspective, the aim in this study was to use tests that were feasible and easy to apply without complicated equipment.
In the static balance test (31) the patients stood on one leg with their eyes open and arms relaxed by their sides. They placed the heel of the opposite foot against the medial side of the supporting leg at the level of the knee joint, and kept the thigh rotated outwards. The uppermost limit for the trial was 60 s and the time was measured in seconds on a stopwatch. If this limit was not reached during the first trial, a second trial was allowed. The better result of 2 trials was used in the statistical analyses. The test was performed separately on each leg, starting with the right leg.
The first test of dynamic balance involved tandem-walking forwards (32). The patients were instructed to place 1 foot in front of the other with the heel and toe of their shoes touching (tandem step), and to walk as quickly as possible along a line 6 m long without touching the sides or making mistakes in the tandem steps. The test was performed 3 times and the walking time for each trial was measured in seconds. The best result of the 3 trials was used in the analyses.
The second dynamic balance test involved tandem-walking backwards (31). The instructions were the same as in the first test, but the walking direction was backwards. The best result of 3 trials was used in the analyses.
As a test of speed of whole body movement and agility the patients were asked to run as fast as possible in a figure-of-8 (33). The course was marked with 2 traffic cones placed 10 m apart, with the start/finish line next to 1 of the cones. The stopwatch was started on the starting signal and was stopped when the subject completed the course and crossed the start/finish line again. The time was recorded in seconds. The test was performed 3 times with a short rest-period between each trial. The best result of the 3 trials was used in the analyses.
The Rhythm co-ordination test (32) consisted of slow and fast phases. The slow rhythm comprised 2 consecutive parts, each lasting 30 s, and the tester scored the performance on each part in points. The patient was asked first to march on the spot in time to a metronome signal (92 beats/min), 1 step for every single beat for 30 s, and then to continue marching for another 30 s and to clap his hands together on every other beat. Points were given for both parts separately according to: (i) accuracy in the first 10 s: 0 = totally asynchronous marching, 1 = gradually getting into the marching rhythm during the first 10 s, 2 = a synchronous marching rhythm at the first attempt; and (ii) maintenance of the exact rhythm from 10 to 30 s: 0 = totally asynchronous rhythm co-ordination while marching and clapping, 1 = difficulties in keeping to the rhythm, 2 = maintaining an accurate marching and clapping rhythm for the rest of the test. The sum of the scores in the slow rhythm phase was thus 0–8 points.
The fast rhythm phase (138 beats/min) started immediately after the slow phase. The same procedure was repeated to the rhythm of the metronome. The sum of the scores in this phase was also 0–8. Both the slow and fast phases were performed only once. The sum of both rhythm test scores (0–16 points) was calculated and used in the analyses.
Statistical analyses
The means, standard deviations (SD), medians, ranges and frequencies are presented as descriptive statistics. Spearman’s partial rank correlation coefficients were used to identify the associations between the continuous neuropsychological tests and the dynamic-balance, agility and rhythm-co-ordination tests. Rank correlations were used due to non-normality in most neuropsychological and motor-performance variables. Adjustment was made for possible confounding variables: age, length of education (years), post-traumatic amnesia and time from injury (months). Ranks rather than original values were used in computing the partial correlation coefficients and their 95% confidence intervals (CI) in all variables. Scatter-plots of the original values are presented for variables with the highest correlations.
The results of the following neuropsychological tests were dichotomized into categories of normal and abnormal performance: all the CERAD subtests except word-list-learning and copying figures (25, 26), and the subtests of the LNI (motor functions and acoustico-motor organization) (27, 28). The distributions of the motor-performance test results in the subcategories (normal/pathological) of neuropsychological tests are described as box-and-whisker plots. Analyses of covariance, adjusted for the same confounders as mentioned above, were used to study the differences in dynamic balance, agility and rhythm-co-ordination between the normal and the pathological results in the neuropsychological tests. The distributions of the dynamic-balance and agility test variables were positively skewed, thus the underlying assumptions of normality and equal group variance were not fulfilled. In order to achieve closer agreement the variables were log transformed for the analyses. Adjusted geometric mean ratios (GMR) were calculated as antilogs of the mean between-group differences in the log-transformed variables. GMR describes the relative difference in group means, a value of one indicating that there is no between-group difference. The sum score of the rhythm-co-ordination tests was used in the original scale and the result presented as an adjusted between-group mean difference. The 95% CIs of the GMRs and the mean differences are also presented as an indication of the precision of the estimates.
Some of the variables in the analyses of the neuropsychological tests were combined because of a high correlation with each other or a low frequency of abnormal results. The LNI motor function subtests used in this study comprised 4 tasks of simple forms of praxis and 2 tasks of complex forms. These tasks, all measuring praxis of the upper limbs, were first analysed as separate neuropsychological test variables, but then combined because of their low frequency.
The results of the static balance tests were dichotomized into categories of 60 s and below 60 s. Logistic regression analysis was used to examine the associations between the neuropsychological tests and the static balance tests when the neuropsychological variables were considered to be continuous, and Fisher’s exact test when they were categorical (normal/pathological). STATA statistical software version 10 was used for the analyses.
RESULTS
The study population is described in Table I. All the participants were men with post-acute TBI who had made a good physical recovery.
The means and SDs of the dynamic balance and agility tests are shown in Table II. In order to measure static balance the patients were asked to stand on the right and left leg in turn: almost half of them were unable to maintain their balance on 1 leg (44% on the right, 50% on the left leg) for 60 s. In the slow phase of the rhythm co-ordination test 41% of the patients had difficulty in starting and/or maintaining the given rhythm, and 62% had difficulty with co-ordination during the fast phase.
|
Table II. Results of neuropsychological and motor performance tests |
|||
|
Tests |
Maximum score or retaining % /Cut-off score (CERAD) |
Frequencies |
Mean (SD) [range] |
|
Neuropsychological tests |
|
Normal/pathological |
|
|
CERAD battery (n = 34) |
|||
|
Verbal fluency (naming animals) |
–/<15 |
29/5 |
|
|
15-item Boston Naming Test |
15/<11 |
32/2 |
|
|
MMSE |
|||
|
Screening score |
30/<25 |
34/0 |
27.7 (1.7) [25–30] |
|
Memory |
|||
|
Word list learning |
30/– |
19.6 (3.4) [13–26] |
|
|
Word list recall |
10/–100/<80 |
26/8 |
|
|
Word list recognition |
20/–100/<80 |
33/1 |
|
|
Constructional praxis |
|||
|
Drawing (a clock) |
6/<5 |
31/3 |
|
|
Recall of constructional praxis |
11/–100/<60 |
30/4 |
|
|
Copy (figures) |
11/– |
|
10.7 (0.9) [7–11] |
|
Trail Making Test, s |
|
|
|
|
Trial A (n = 34) |
50.5 (24.1) [23–145] |
||
|
Trial B (n = 33) |
125.8 (60.8) [54–310] |
||
|
WAIS-R (sub-test) (n = 34) Digit symbol |
41.9 (11.1) [25–77] |
||
|
LNI: motor functions and acoustico-motor organization (sub-tests) (n = 33) |
|||
|
Motor functions of hands |
|||
|
Simple forms of praxis |
20/13 |
||
|
Complex forms of praxis |
29/4 |
||
|
Dynamic organization |
16/17 |
||
|
Speech regulation of motor act |
30/3 |
||
|
Acoustico-motor organization |
|||
|
Perception of rhythmic structures |
27/6 |
||
|
Reproduction of rhythmic structures |
17/16 |
||
|
Motor Performance tests |
|
|
|
|
Dynamic balance (n = 34) |
|||
|
Tandem walking forwards, s |
14.9 (4.3) [9.3–28.5] |
||
|
Tandem walking backwards, s |
17.6 (6.3) [10.0–37.2] |
||
|
Agility (n = 34) |
|||
|
Running in figure-of-8, s |
8.4 (2.1) [6.2–15.6] |
||
|
Unsuccessful/successful |
|||
|
Static balance (n = 34) |
|||
|
Balance on the right leg, < 60 s/60 s |
15/19 |
||
|
Balance on the left leg, < 60 s/60 s |
17/17 |
||
|
Rhythm-co-ordination (n = 34) |
|||
|
Slow rhythm, 0–6 points/7–8 points |
14/20 |
||
|
Fast rhythm, 0–6 points/7–8 points |
21/13 |
||
|
MMSE: Mini Mental State Examination; WAIS-R: Wechsler Adult Intelligence Scale Revised; LNI: Luria’s Neuropsychological Investigation; CERAD: Consortium to Establish a Registry for Alzheimer’s Disease. |
|||
The neuropsychological sequelae of the study patients consisted, in general, of various combinations of problems, including fatigue, slowness of information processing, disorders in attention and memory, disturbances in executive skills and modulation of affective states, likewise disorders in language communication. The neuropsychological test results are shown in Table II.
Correlations between the continuous neuropsychological and motor-performance test results are shown in Table III. The highest rank correlations were between the time for both Trail Making tests and the performance time for Running a figure-of-8 (rs = 0.57). Moreover, the scores on the Digit Symbol test correlated inversely with the latter (rs = –0.52; 95% CI –0.74 to –0.19). The original values behind these highest rank correlations are shown in scatter-plots in Fig. 1. Other correlations between these variables were only weak or moderate (< 0.35).
|
Table III. Correlations between neuropsychological and motor performance test results (Spearman’s partial rank correlation coefficients) |
||||
|
Tests |
Tandem walking forwards, s rs (95 % CI) |
Tandem walking backwards, s rs (95 % CI) |
Running figure-of-8, s rs (95 % CI) |
Rhythm test, score rs (95 % CI) |
|
CERAD, score (n = 33) |
||||
|
MMSE Screening |
0.15* (–0.23 to 0.49) |
0.19 (–0.19 to 0.52) |
0.11 (–0.27 to 0.46) |
–0.12 (–0.46 to 0.26) |
|
Memory Word list learning |
–0.17 (–0.51 to 0.21) |
–0.31 (–0.61 to 0.07) |
–0.33 (–0.62 to 0.04) |
–0.05 (–0.41 to .33) |
|
Trail Making Test, s |
||||
|
Trial A (n = 33) |
0.34 (–0.03 to 0.63) |
0.23 (–0.15 to 0.55) |
0.57 (0.26 to 0.78) |
–0.23 (–0.55 to 0.15) |
|
Trial B (n = 32) |
0.33 (–0.05 to 0.63) |
0.26 (–0.13 to 0.58) |
0.57 (0.25 to 0.78) |
–0.32 (–0.62 to 0.06) |
|
WAIS-R, sub-test, score (n = 33) Digit symbol |
–0.37 (–0.65 to 0.00) |
–0.18 (–0.51 to 0.20) |
–0.52 (–0.74 to –0.19) |
0.01 (–0.36 to 0.37) |
|
*Spearman’s partial rank correlation coefficient adjusted for age, length of education, post-traumatic amnesia and time from injury. CERAD: Consortium to Establish a Registry for Alzheimer’s Disease; CI: confidence interval; MMSE: Mini Mental State Examination; WAIS-R: Wechsler Adult Intelligence Scale Revised. |
||||
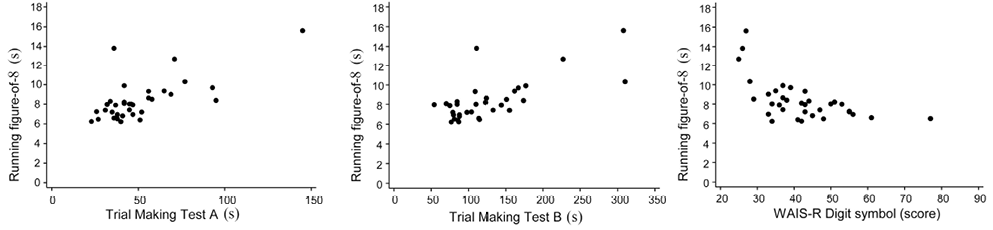
Fig. 1. Scatter-plots between neuropsychological tests (Trail Making and Wechsler Adult Intelligence Scale Revised (WAIS-R) Digit symbol tests) and Running-figure-of-8.
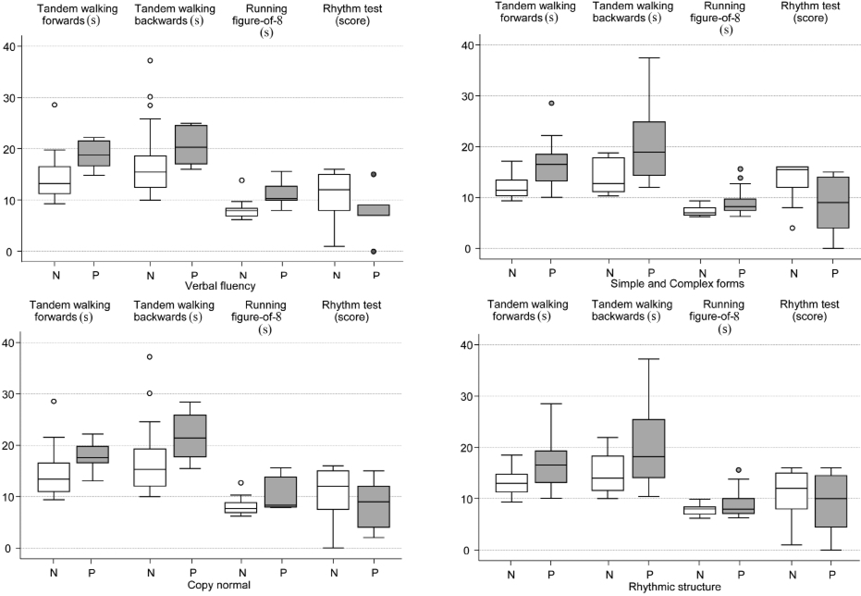
The distributions of the motor-performance test results in subcategories of categorized neuropsychological tests are shown in Fig. 2, and the adjusted between-group differences in Table IV. The group of patients with normal results in verbal fluency achieved a 26% faster mean performance time in Tandem walking forwards (GMR 0.74; 95% CI 0.55 to 1.00) and Running a figure-of-8 (GMR 0.74; 95% CI 0.61 to 0.89) compared with those with abnormal fluency. In addition, patients with a normal result in the reproduction of rhythmic structures produced, on average, 20% and 23% better performance times in Tandem walking forwards (GMR 0.80; 95% CI 0.65 to 1.00) and Tandem walking backwards (GMR 0.77; 95% CI 0.59 to 1.00), respectively. Motor functions of the hands (simple and complex forms of praxis) correlated significantly with all these motor-performance tests. No statistically significant associations were found between the neuropsychological test results and static balance. Moreover, dynamic forms of praxis, speech regulation of motor acts and the perception of rhythmic structures showed no associations with any of the motor-performance test results.
Fig. 2. Box-and-whisker plots of motor-performance tests according to groups of the categorized neuropsychological tests. The horizontal line inside the box represents the median and the bottom and top of the box are the lower and upper quartiles, respectively. The lines and dots outside the box represent the values outside the inter-quartile range. N = normal; P = pathological test result.
|
Table IV. Differences in motor performance tests between normal and pathological values in neuropsychological tests |
||||||
|
Tests |
Tandem walking forwards, s Mean (SD) |
Tandem walking backwards, s Mean (SD) |
Running figure- of-8, s Mean (SD) |
Rhythm test, score Mean (SD) |
||
|
CERAD |
||||||
|
Verbal fluency Normal (n = 28) Pathological (n = 5) Geometric mean ratioa |
14.3 (4.2) 18.8 (3.1) 0.74 (0.55 to 1.00) |
17.1 (6.7) 20.6 (4.2) 0.83 (0.57 to 1.20) |
7.9 (1.5) 11.3 (2.9) 0.74 (0.61 to 0.89) |
Mean diff.a |
10.8 (5.2) 7.6 (5.4) 3.3 (–2.5 to 9.2) |
|
|
Constructional praxis: copy Normal (n = 27) Pathological (n = 6) Geometric mean ratioa |
14.3 (4.3) 17.8 (3.1) 0.77 (0.58 to 1.03) |
16.7 (6.4) 21.7 (5.3) 0.75 (0.53 to 1.06) |
8.0 (1.5) 10.3 (3.5) 0.82 (0.67 to 1.01) |
Mean diff.a |
10.7 (5.4) 8.5 (5.0) 2.8 (–2.9 to 8.5) |
|
|
LNI: motor functions and acoustico-motor organization (sub-tests) |
||||||
|
Simple and complex forms of praxis Normal (n = 11) Pathological (n = 21) Geometric mean ratioa |
11.9 (2.4) 16.3 (4.4) 0.74 (0.60 to 0.90) |
13.4 (3.2) 19.8 (6.8) 0.69 (0.54 to 0.88) |
7.2 (1.0) 9.0 (2.4) 0.81 (0.70 to 0.95) |
Mean diff.a |
13.6 (4.1) 8.5 (5.2) 5.9 (2.0 to 9.7) |
|
|
Reproduction of rhythmic structures Normal (n = 16) Pathological (n = 16) Geometric mean ratioa |
13.1 (2.9) 16.6 (4.9) 0.80 (0.65 to 1.00) |
14.9 (3.9) 20.3 (7.6) 0.77 (0.59 to 1.00) |
7.8 (1.2) 9.0 (2.8) 0.93 (0.79 to 1.10) |
Mean diff.a |
11.2 (5.2) 9.4 (5.6) 2.6 (–1.7 to 7.0) |
|
|
aGeometric mean ratio (95% confidence interval (CI)) or mean difference (95% CI) between categories of neuropsychological tests (normal vs pathological) are estimated by analysis of covariance and they are adjusted for confounders (age, length of education, post-traumatic amnesia and time from injury). The difference is expressed as geometric mean ratio for log-transformed dependent variables and as mean difference for dependent variable without transformation. |
||||||
DISCUSSION
The aim of this explorative study was to evaluate the relationship between cognitive and motor-performance in physically well-recovered men with significant TBI in the post-acute stage. Analyses of the relationships between the neuropsychological and motor-performance tests showed associations between the speed of complex information processing and attention (the Trail Making and Digit Symbol tests), and performance time in agility (Running a figure-of-8). Moreover, patients with normal performance in the measures of executive functioning (verbal fluency and reproduction of rhythmic structures) also produced a faster mean performance time in tests of dynamic balance and/or agility (Tandem walking forwards/backwards and Running a figure-of-8) than those with abnormal executive functioning. Thus, fluency of information processing and executive functioning was reflected in the speed of walking and running, and vice versa. Motor functions of the hands (simple and complex forms of praxis) also correlated with the results of all motor-performance tests except static balance.
These findings concur with those reported in earlier studies indicating that measures of information processing, attention and executive functioning may be associated with locomotor behaviour (18–20). Cantin and co-workers (19) found that during locomotor activities, subjects with TBI walked more slowly, had higher clearance margins and longer reading times in the Stroop tasks (attentional flexibility and speed of information processing) (27) than healthy subjects. Furthermore, they observed significant relationships between scores on Trail Making B (attention, complex information processing, visual conceptual, visuomotor tracking and cognitive flexibility) and clearance margins among people with TBI, but not among healthy subjects. According to the authors this may have been the result of poor planning ability because the TBI subjects who performed poorly on the Trail Making B test showed higher clearances over the obstacles in complex environments. These findings support the hypothesis that certain measures of cognitive functioning may help to predict motor performance in complex environments following TBI.
The patients in the present study had difficulty in maintaining static balance on both the right and the left leg. Furthermore, 41% of them had difficulty while simultaneously marching and clapping hands to the slow rhythm, and the fast-rhythm co-ordination task gave them even more problems (62%). These findings are in line with those of other studies indicating that balance and more complex motor tasks involving co-ordination are common functional deficits after TBI (7–11). Azouvi et al. (34) also reported slowed information processing in a dual-task test and difficulties under high time-pressure in laboratory settings.
Tandem-walking tests were used to measure dynamic balance in the present study, and the performance time was associated with the measures of executive functioning. As a consequence, the performance time of the balance test turned out to be more revealing than the balance control per se (performing the test without side touches or mistakes in tandem steps). The time measure in the tandem-walking test has not generally been considered so important. Nevertheless, it may bring a whole new dimension to the evaluation via the identified link to information processing and executive functioning.
The finding in this study that the motor functions of the hands correlated with almost all motor-performance tests may be due to the fact that the motor component was rigorously tested. On the other hand, the tests of hand motor functions included simple and complex forms of praxis, referring to executive functions and their role in producing voluntary movements. The hand praxis correlated with all the motor-performance tests except static balance, which may also indicate the importance of the executive component in the motor functions in question.
The present study showed no correlation between the reproduction of rhythmic structures and rhythm-co-ordination on the motor side. Some interaction might have been expected based on the regulation of motor acts (executive functions), which is needed in both tasks for producing voluntary movements. One reason for the absence of correlation may have been the differences in content and demands on regulation (executive function) in the two tasks: reproducing rhythms from a pattern presented acoustically vs marching on the spot in time with a metronome signal and clapping the hands.
Serrien et al. (15) attempted to model the neural processes recruited during complex motor tasks. According to them, a network comprising primary and secondary sensorimotor areas, as well as subcortical regions, is engaged during well-learned motor skills. Cognitive resources are recruited during the acquisition of complex skills and when external and internal factors are altered. Their function is to ensure that action is performed in accordance with the goal requirements, and that frontal lobe systems linked to response selection and monitoring are engaged. As in the model developed by Serrien et al., our findings show that, in practice, cognitive processes are related to motor processes. Although the significance of such processes is generally accepted, their underlying mechanisms and interaction with motor circuits are far from clear.
Apart from the theoretical relevance of the study, the finding of an association between cognitive and motor performance may have potential clinical relevance with regard to rehabilitation. The acknowledged benefits of physical conditioning for people with TBI include improved sleep patterns, reduced fatigue, stronger endurance, reduced depression, increased self-confidence and improved individual autonomy (35, 36). Furthermore, physical activity and exercise can improve cognitive functioning and concentration after brain injury through engagement in a pleasant activity (37). Thornton et al. (38) found that both an activity-based and a virtual balance exercise programme for adults with TBI offered benefits over and above improved balance. The virtual-reality group gave more comments expressing enjoyment and improved confidence, and reported that their day had more structure and purpose. Combining therapeutic cognitive and motor activities may approximate the demands of everyday life more closely than artificially separating them in separate therapy sessions. It seems that if rehabilitation is to be successful it is crucial to address problems from multiple perspectives and to foster comprehensive and trans-disciplinary teamwork (5, 21). Furthermore, exploring the relationship between action and cognition might support the design of cognitive interventions that emphasize strategic and evaluative operations in order to improve behavioural performance after brain injury.
The results of this study indicate a relationship between cognitive and motor performance, but no causation can be assumed. An important direction for the future research would be to study the possible causalities between these factors: for example, does improvement in cognitive functioning following a treatment programme correlate with improvements in motor functioning, and vice versa?
Some limitations of this study should be acknowledged. First, the study was conducted among men, and generalization to women with regard to the evaluation of motor performance requires caution. In general, men are at higher risk of TBI (the risk for men is 0.88–2.5 times higher than for women) (39). Secondly, the patients with TBI were fairly heterogeneous with respect to GCS scores (range 3–15) and types of CT/MRI findings. On the whole, they seemed to have recovered well physically, which was consistent with the inclusion criteria. Thirdly, the representativeness of the results is limited due to the small sample size. Nevertheless, the number of patients was sufficient for reliable explorative statistical analysis and the results can be interpreted as indicative.
In conclusion, the results show that measures of information processing, attention and executive functioning may be associated with motor performance. The findings support the interaction between motor performance and cognition as reported in the literature. However, further research with larger sample sizes, including both sexes and different subgroups of patients with TBI, is needed in order to confirm the precise nature of the connection. A relevant goal for future research would be to use techniques such as functional magnetic resonance imaging and diffusion tensor imaging in order to ascertain how cognitive and motor pathways are connected neurally. The finding of an association between cognitive and motor performance may also have potential clinical relevance with regard to developing comprehensive neurorehabilitation. However, further research is needed before more definite clinical implications can be drawn.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the support of the Ministry of Education, Finland Grant No. 196/722/2000 and the Medical Fund of Helsinki and Uusimaa Hospital District. The authors would like to thank Jukka Turkka, MD, PhD, for his expertise in confirming the injury-related variables of the patients and for his review of the manuscript; Sanna Koskinen, PhD, for her helpful advice and comments while reviewing the manuscript, and all the participants for their collaboration in this study. The authors express their deepest gratitude and honour the memory of their senior co-author, Professor Hannu Alaranta, who passed away on 10 June 2008.
REFERENCES
