Ela Tarakci, PhD, PT1, Ipek Yeldan, PhD, PT1, S. Nilay Baydogan, MSc, PT2, Seref Olgar, MD3 and Ozgur Kasapcopur, MD4
From the 1Division of Physiotherapy and Rehabilitation, Faculty of Health Sciences, Istanbul University, 2Center of Burcu Physical Therapy and Rehabilitation, Levent, Istanbul, 3Division of Pediatric Cardiology, Medical Faculty,
Kahramanmaras Sutcu Imam University and 4Department of Pediatric Rheumatology, Medical Faculty of Cerrahpasa, Istanbul University, Turkey
OBJECTIVE: To investigate the effects of a land-based home exercise programme on pain, functional ability and quality of life in patients with juvenile idiopathic arthritis.
DESIGN: A randomized, controlled, single-blind study.
Patients: Eighty-one patients with juvenile idiopathic arthritis participated in this study.
METHODS: Functional ability, pain, and quality of life were assessed with a 6-minute walk test, Childhood Health Assessment Questionnaire, visual analogue scale, and the Pediatric Quality of Life Inventory. The patients were randomly assigned to an exercise or control group. The exercise group (n = 43) completed a 12-week individually planned land-based home exercise programme once a week at the hospital for 4 days per week. The control group (n = 38) was placed on the waiting list until the end of the study.
RESULTS: Statistically significant improvements (p < 0.001) were found in all the outcome measures in the exercise group. The visual analogue scale score decreased significantly (p < 0.01) in the control group after 12 weeks. Other than the visual analogue scale score (p > 0.05), the changes in the other outcome measures (p < 0.001) were significant in favour of the exercise group.
CONCLUSION: The study demonstrated that participating in a 12-week individually planned land-based home exercise programme may result in improved physical function and quality of life in patients with juvenile idiopathic arthritis.
Key words: juvenile idiopathic arthritis; exercise; pain; functional ability; quality of life.
J Rehabil Med 2012; 00: 00–00
Correspondence address: Ela Tarakci, Istanbul Universitesi, Saglik Bilimleri Fakultesi, Fizyoterapi ve Rehabilitasyon, Bolumu, Capa Kampusu, TR-34452, Fatih, Istanbul, Turkey. E-mail: etarakci@istanbul.edu.tr
Submitted February 29, 2012; accepted June 19, 2012
INTRODUCTION
Juvenile idiopathic arthritis (JIA) is a common chronic illness that affects 1 in 1,000 children (1). Patients with JIA may experience significant disability due to muscular weakness, joint pain, contracture, and reduced mobility. Children with JIA participate in fewer physical activities and have lower functional ability and decreased physical fitness compared with their peers (2–5).
The aim of JIA treatment is to control the disease, preserve the physical and psychological integrity of the child, and prevent any long-term consequence related to the disease or its therapy (6). Physical therapy for patients with JIA aims to manage pain and inflammation, preserve range of motion (ROM), and maintain muscle strength through rest and limiting the strain on arthritic joints (7, 8). Guidelines have included recommendations for fitness and strengthening exercise in children with JIA to improve function and promote lifetime physical activity (9, 10). Exercise interventions result in improved aerobic capacity, muscle strength, and disease activity, with a possible beneficial effect on pain, function, and quality of life (11–13). However, the best therapeutic exercise programme for children with JIA is unknown. Previous studies have indicated that children with JIA derive some benefit from an aquatic exercise programme, which did not lead to worsening health status (14–16). On the other hand, one study showed that land-based exercise may improve physical fitness in JIA patients (17).
Whether exercise influences functional ability and health-related quality of life in JIA patients is relatively unknown. Our primary focus related to exercise training in JIA was to improve patient-centred outcome measures. Therefore, the aim of the current study was to investigate the effects of an individually planned land-based home exercise (LBHE) programme on pain, functional ability, and quality of life, using a randomized, controlled, single-blind design.
METHODS
A total of 81 patients with JIA (44 girls and 37 boys), age range 5–17 years, participated in this study. The enrolment period was between July and October 2011. The patients were recruited from the pediatric rheumatology outpatient clinic of the Department of Pediatric Rheumatology of the Istanbul University Faculty of Cerrahpasa Medicine. JIA was diagnosed in accordance with the International League of Associations for Rheumatology (ILAR) criteria (18). Because our LBHE programme is performed under parental supervision at home, we considered the presence of active joints in the exacerbation period to be an exclusion criterion. The other exclusion criteria were neurological disease, metabolic disorder, decompensated organ failure, intra-articular steroid injection or surgery in any joint, > 2 h habitual regular weekly exercise (aerobic exercises such as swimming/cycling, callisthenic exercise, or strengthening exercise), and if they were unable to cooperate with exercise or measurement. All the patients were asked to maintain a stable dosage and continue taking their prescribed medical treatment regularly throughout the study. The patients and their parents were informed about the study and written informed consent was obtained from the parents of the patients. The study was approved by the ethics committee of Istanbul University and was conducted in accordance with the Declaration of Helsinki.
The demographic data were reported by a physical therapist. The patients’ weights and heights were measured, and the body mass index (BMI) was calculated as the weight divided by the square of the height. The clinical features, such as disease duration, subtype, and the number of active joints, were assessed by a paediatric rheumatologist.
Sample size calculation
The estimated sample size required to detect a statistically significant difference between the LBHE and control groups, at a 5% significance level with a power of 90%, was 42 patients. A 4% reduction (0.13 of the total score of 3) in the Childhood Health Assessment Questionnaire (CHAQ) score was estimated using the data from the study of JIA patients by Dempster et al. (19).
Study design
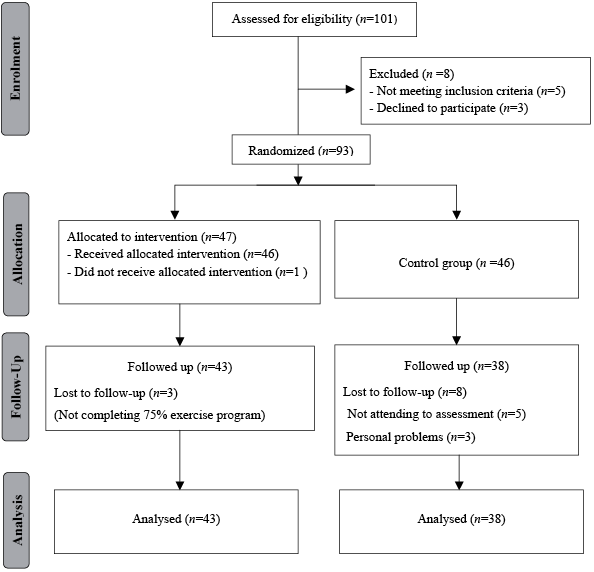
The participants were randomly assigned to the exercise or control group. The randomization was performed using the Microsoft Excel [RAND function; =IF(RAND()<=0.5; “LBHE group”; “control group”)]. We used concealed allocation in the randomization. A flow chart is presented in Fig. 1. The exercise group (n = 43) completed a 12-week LBHE programme. The control group (n = 38) was assigned to a waiting list until the end of the study. All measurements were performed before and after the 12 weeks. The selector (IY), who did not perform any assessment, was aware of the randomization scheme. The assessor (ET) was blind to which group the patients had been allocated and applied a standard procedure in both groups. The treating physical therapist (SNB) was aware of the nature of this intervention and the physical findings of the patients with JIA, but was blind to the assessments.
Fig. 1. Progress of study participants.
Outcome measures
Six-minute walk test (6-MWT). The 6-MWT has been used as a measure of the functional status of patients (20). Lelieveld et al. (21) had provided support for the validity of the 6-MWT for patients with JIA. The test was performed on an 8-m track in a straight corridor, as described in the literature. The patients were instructed to cover the largest possible distance in 6 min at a self-chosen walking speed. The turns were made on both ends of the 8-m track. The distance walked was recorded with a lap counter. Each time the patient returned to the starting line, the lap counter clicked once. Every minute, the patients were encouraged in a standardized manner and at a standardized time, recorded with a stopwatch. At the end of the test, the patient was asked to stand still, and the distance covered in the final partial lap was measured. This was performed with a measuring tape. The total distance covered was calculated by multiplying the number of laps by 16 m and adding the additional metres in the final partial lap (22). The total outcome of walking distance was recorded for analysis.
Functional ability: Child Health Assessment Questionnaire (CHAQ). CHAQ is the most widely used functional health status measure in children with JIA (23). Functional ability was assessed by the Turkish version of CHAQ, which is a reliable and valid tool for the functional, physical, and psychosocial assessment of children with JIA (24). In 8 domains (dressing/grooming, arising, eating, walking, hygiene, reach, grip, and activities), a number of questions were answered and scored on a scale of 0–3, where 0 represents the ability to perform with no difficulty; 1, the ability to perform with some difficulty; 2, the ability to perform with much difficulty; and 3, the inability to perform. When assistance or aids were required for a domain, the score for that domain was raised to a minimum of 2. The time period for the self-assessment was 1 week. The mean of the 8 scores determined the CHAQ score (range: 0–3).
Pain. The CHAQ also provided an assessment of discomfort using a 100-cm visual analogue scale (VAS) for the evaluation of pain (23). A score of 0 indicated “no pain,” and a score of 100 represented “extreme pain” on the VAS.
Quality of life: The Pediatric Quality of Life Inventory Arthritis Module (PedsQL). The PedsQL 3.0 arthritis module was designed by Varni (25) to measure health-related quality-of-life dimensions specifically tailored to pediatric rheumatology. We used the Turkish version of the PedsQL 3.0 arthritis module, which is a reliable and valid tool for quality of life assessment in children with JIA (26). The 22-item multidimensional PedsQL 3.0 arthritis module encompasses the following scales: pain and hurt (4 items), daily activities (5 items), treatment (7 items), worry (3 items), and communication (3 items). Patients were asked to determine to what extent each item has been a problem during the past one month. A 5-point Likert scale was used for the patients’ self-reporting (age range 8–18 years) or the parents’ proxy report (0: never a problem, 1: almost never a problem, 2: sometimes a problem, 3: often a problem, and 4: almost always a problem). To further increase the ease of use for young children’s self-reporting (age range 5–7 years), the Likert scale is re-worded and simplified to a 3-point scale (0: not at all a problem, 2: sometimes a problem, and 4: a lot of a problem), with each response choice anchored to a scale of happy to sad faces. The parents’ proxy report also covers the toddler age range (2–4 years), which does not include a self-reporting form, given the developmental limitations of self-reporting for children younger than age 5 years. The proxy form includes only 3 subscales: pain and discomfort, daily activities, and treatment (27, 28).
Intervention
The LBHE programme was designed individually for the exercise group by a physical therapist. The exercise programme was planned to improve strength, flexibility, functional ability, and quality of life, and to encourage more frequent use of the affected extremity in a controlled manner.
Patients were given an individual exercise programme that included ROM, strengthening, stretching, and posture exercises that were performed daily at home. The ROM exercises were applied to affected joints. In the earlier weeks, the exercise programme included active assistive or active ROM exercises. At a later week, the exercise was shifted toward active or active resistive ROM exercises. The strengthening exercises were performed for upper and lower extremity muscles using a Theraband (The Hygenic Corporation, Akron, OH, USA). Stretching was performed with moderate tension and duration of 20–30 s. Pectorals, hamstrings, hip flexors, and the Achilles tendon were included in the stretching exercises. A postural exercise was also included in the exercise programme. Scapular muscles and back extensors were trained to prevent a round shoulder. Functional activities, such as walking, squats, and stair climbing, were also included in the exercise programme.
Patients were supervised once a week by physical therapists in the hospital. The exercises were performed daily for 3 days as a home programme under parental supervision. The number of exercises was initially restricted to produce compliance to the exercise, and the impact was applied to most affected joints. The number of repetitions and the difficulty were increased gradually; the maximum number of repetitions was 15. The duration of the exercise session was a minimum of 20 min and a maximum of 45 min, with this duration gradually increasing from minimum to maximum during the study (Table I). The exercises were demonstrated to all of the patients by the same physical therapist. Progression in the LBHE programme was provided according to each patient’s tolerance and response to the exercise programme. The patients and their parents were instructed about joint protection and the exercise programme by the physical therapist. To promote compliance with the therapy, the patients were asked to write a diary of the exercise programme, which was reviewed weekly. The data of the patients who completed more than 75% of the exercise programme were included in the outcome measures after 12 weeks.
|
Table I. Outline of individual land-based home exercise programme |
|
|
Parameter |
Description |
|
Warm-up |
Active assistive or active range of motion exercises |
|
Strengthening exercises |
Active resistive ROM exercises with Theraband Muscle groups: gluteus medius, gluteus maximus, iliopsoas, quadriceps femoris, hamstrings, tibialis anterior, deltoid, triceps, biceps, forearm muscles, hand muscles |
|
Stretching exercises |
Pectorals, hamstrings, hip flexors, tensor facia lata, Achilles tendons (moderate tension and duration of 20–30 s) |
|
Postural exercises |
Rhomboids, lower and middle trapezius, latissimus dorsi, serratus anterior, and back extensors training |
|
Functional activities |
Walking, squat and stair-climbing |
|
Repetition |
1 set of 8–10 repetitions, increase gradually to 10–15 repetitions (for strengthening) 1 set of 3 repetitions, increase gradually to 5 repetitions (for stretching) |
|
Duration |
20–45 min |
|
Frequency |
1 day/week under physical therapist’s supervision at hospital 1 session/day, 3 day/week under parents’ supervision at home |
The patients in the control group were enrolled in the waiting list until the end of the study. These patients were interviewed by telephone once a month and received information about their clinical status.
In both groups, all the patients were asked to maintain a stable dosage of medication and to continue their usual physical activity level throughout the study.
Statistical analysis
The data were evaluated using the Statistical Package for Social Science (SPSS) v.17.0 software for Windows and by analysing descriptive statistics (frequency, mean, and standard deviation (SD)). We performed a power analysis to determine the sample size at the beginning of the study, using the Raosoft sample size calculator. Before the statistical analysis, all the variables cohered to the normal distribution (p > 0.05) according to the normal probabilistic graph and Kolmogorov-Smirnov test. An independent samples t-test was used to determine the differences in the subjects’ demographic and clinical features because the data were distributed normally. A paired samples t-test was used to determine the effects of the exercise programme. A significance level of p < 0.05 was used.
RESULTS
A total of 93 eligible patients with JIA were included in this randomized, controlled, single-blind study. Four patients left the exercise group, and 8 patients left the control group before the end of the study, leaving a total of 81 patients who completed our study. In the exercise group, the patient population comprised 27 patients with polyarticular JIA, 14 patients with oligoarticular JIA, 1 with systemic arthritis, and 1 with psoriatic arthritis. In our control group, the patient population consisted of 19 patients with polyarticular, 16 with oligoarticular, and 3 with systemic arthritis.
The demographic and clinical features of the two groups are shown in Table II. The mean age was 10.02 years (SD 3.44; range 5–17 years) in the exercise group and 10.82 years (SD 4; range 5–16 years) in the control group. The mean disease duration at the time of the enrolment was 5.31 years (SD 3.05; range 1–13 years) in the exercise group and 6.50 years (SD 3.83; range 1–15 years) in the control group. The analysis indicated no significant differences in demographic or clinical background between the two groups.
|
Table II. Comparison of demographic and clinical features between 2 groups |
|||
|
Demographic/clinic features |
Exercise group n = 43 |
Control group n = 38 |
p-value |
|
Age, years, mean (SD) |
10.02 (3.44) |
10.82 (4.00) |
0.34 |
|
BMI, kg/m2, mean (SD) |
17.28 (3.52) |
18.22 (3.60) |
0.23 |
|
Sex, F/M, n |
25/18 |
19/19 |
0.46 |
|
Count of affected joint, n |
3.42 (1.25) |
3.55 (2.15) |
0.73 |
|
Disease duration, years, mean (SD) |
5.31 (3.05) |
6.50 (3.83) |
0.12 |
|
JIA subtype, n (%) |
|
|
|
|
Polyarticular |
27 (62.8) |
19 (50) |
|
|
Oligoarticular |
14 (32.6) |
16 (42.1) |
|
|
Systemic |
1 (2.3) |
3 (7.9) |
0.35 |
|
Psoriatic |
1 (2.3) |
0 |
|
|
Affected joint, n (%) |
|
|
|
|
Knee |
24 (55.81) |
21 (55.26) |
|
|
Hip |
7 (16.27) |
4 (10.52) |
|
|
Ankle |
6 (13.95) |
7 (18.42) |
|
|
Wrist |
4 (9.32) |
6 (15.8) |
|
|
Shoulder |
2 (4.65) |
0 |
|
|
SD: standard deviation; BMI: body mass index; F: female; M: male; JIA: juvenile idiopathic arthritis. |
|||
The comparison of the initial assessment values for 6-MWT, CHAQ, VAS, PedsQL self-report, and PedsQL parental proxy report between the groups is presented in Table III. There was no statistically significant difference in outcome measures between the two groups at the start of the study.
|
Table III. Comparison of first assessment values between the 2 groups |
|||
|
Parameters |
Exercise group n = 43 Mean (SD) |
Control group n = 38 Mean (SD) |
p-value |
|
6-MWT, m |
399.47 (97.01) |
436.13 (84.05) |
0.07 |
|
CHAQ |
0.63 (0.67) |
0.66 (0.69) |
0.85 |
|
VAS |
27.67 (23.88) |
36.05 (34.35) |
0.2 |
|
PedsQL-Self Report |
63.58 (25.20) |
61.03 (23.28) |
0.63 |
|
PedsQL-Parent Report |
63.41 (25.49) |
63.95 (24.24) |
0.92 |
|
SD: standard deviation; 6-MWT: 6-minute walking test; CHAQ: Childhood Health Assessment Questionnaire; VAS: visual analogue scale; PedsQL: Pediatric Quality of Life Inventory. |
|||
The changes in outcome measures within and between the two groups at the end of the exercise programme are represented in Table IV. Whereas only the VAS score decreased significantly (p < 0.01) in the control group, statistically significant improvements (p < 0.001) were found in all the outcome measures in the exercise group after 12 weeks. Except for the VAS score (p > 0.05), the changes in the other outcome measures (p < 0.001) were significant, in favour of the exercise group.
|
Table IV. Comparison of changes in outcome measures within and between 2 groups |
|||||||||
|
|
Exercise group |
Control group |
Exercise group |
Control group |
|
||||
|
Pre Mean (SD) |
Post Mean (SD) |
p |
Pre Mean (SD) |
Post Mean (SD) |
p |
Difference Mean (SD) |
Difference Mean (SD) |
p |
|
|
6-MWT, m |
399.47 (97.01) |
430.26 (99.49) 0.19 (0.34) |
0.000 |
436.13 (84.05) |
436 (89.26) |
0.859 |
30.79 (58.85) |
0.63 (21.69) |
0.000 |
|
CHAQ |
0.63 (0.67) |
0.000 |
0.66 (0.69) |
0.64 (0.71) |
0.117 |
–0.43 (0.43) |
–0.02 (0.08) |
0.000 |
|
|
VAS |
27.67 ( 23.88) |
18.26 (23.88) |
0.000 |
36.05 (34.35) |
29.34 (28.45) |
0.002 |
–9.41 (10.53) |
–6.71 (12.58) |
0.29 |
|
PedsQL-Self Report |
63.58 (25.20) |
85.58 (13.31) |
0.000 |
61.03 (23.28) |
62.42 (24.41) |
0.190 |
21.99 (14.23) |
1.39 (6.43) |
0.000 |
|
PedsQL-Parent Report |
63.41 (25.49) |
86.17 (12.48) |
0.000 |
63.95 (24.24) |
65.04 (25.11) |
0.191 |
22.76 (15.32) |
1.09 (5.06) |
0.000 |
|
SD: standard deviation; 6-MWT: 6-minute walking test; CHAQ: Childhood Health Assessment Questionnaire; VAS: visual analogue scale; PedsQL: Pediatric Quality of Life Inventory. |
|||||||||
DISCUSSION
Our study has sufficient power as a randomized, controlled, single-blind trial of LBHE for patients with JIA. We demonstrated that participating in an individually planned 12-week LBHE programme may result in improved physical function and quality of life of JIA patients.
Patients with JIA may experience a limitation in the functioning of one or more joints and stiffness or fatigue as a result of the arthritis. This may have considerable impact on the patient’s daily living activities and quality of life, resulting in an inactive lifestyle (6). Therefore, the main focus of this study was to improve functional parameters, pain, and quality of life. The LBHE and control groups in our study showed significant improvements in VAS score, with no significant difference between the groups. This finding may be a result of the positive effect of medical treatment on pain management in both groups.
The walking distance increased significantly, by 30.79 m in the exercise group, compared with an increase of 0.63 m in the control group. In our patient population, there was high involvement of the lower extremities. For this reason, we designed the exercise programme to include lower extremity functional activities. We considered improvement in walking distance to be a result of our comprehensive exercise programme. As another functional outcome measure, we used CHAQ, a reliable and valid tool to assess functional ability in JIA. Dempster et al. (19) stated that the minimal improvement of clinical importance on the CHAQ assessment is a reduction in the score of 0.13. Our study showed a mean reduction of 0.43 in the LBHE group; thus, this improvement can be considered clinically relevant. In the control group, no significant change in CHAQ score was found after the study. There was also an increase of 21.99 in the self-reported PedsQL score and 22.76 in the parent-reported PedsQL score in the LBHE group. The benefits observed in the PedsQL report might be a result of being involved in a training programme and the added attention received from a physical therapist, or it may be the result of improved functional ability.
In our study, all the outcome measures reported no deterioration in either group. Except for one patient who left the study because of an exacerbation of symptoms, we did not observe worsening of clinical signs or outcome measures in the LBHE group in the short term. This result suggests that our exercise programme is safe and feasible for our patient population. Our exercise programme was individualized for each child, and the components of the exercise programme were determined according to the child’s clinical signs as a supervised home exercise programme. In our study, we took into account some suggestions to improve the patient’s adherence (29, 30). The patients and parents were educated about unexpected responses with respect to exercise training and joint protection. We clearly explained how the recommended exercise programme may help them, and we provided specific information about how to exercise safely and effectively and how to recognize post-exercise soreness. A high adherence to the exercise programme may suggest that our exercise programme is feasible for patients with JIA. To explain our high patient compliance, we thought that it was related to planning an individual exercise programme. The control group maintained their initial functional level and quality of life during the study. In addition, the pain level improved in the control group. Nevertheless, the benefits of performing an individual exercise programme were not observed in the control group with respect to increasing function and quality of life.
Klepper (7) reported that exercise protocols vary in length (6–20 weeks), frequency (1–3 times a week), duration (30–60 min), intensity (60–70% of maximal heart rate), medium (water, land, or combined), and composition (aerobic training, resistance training, general conditioning, and sport-specific training). In our study, we investigated the effects of a 12-week, 20–45-min duration LBHE programme including resistance training and general conditioning. In comparison with the control group, the LBHE group can benefit from engaging in our exercise programme with respect to functional and quality of life measures.
In the literature, we found only 3 randomized controlled trials demonstrating the benefits of exercise training in JIA patients (16, 31, 32). Epps et al. (31) demonstrated an improvement with land-based physiotherapy and a combination of hydrotherapy and land-based physiotherapy, with the patients in the combined group showing greater improvements in physical aspects of health-related quality of life. Their patients received 16-h treatment sessions over a period of two weeks, followed by local physiotherapy attendance for two months in 3 tertiary centres. The land-based physiotherapy programme included stretches, active movements and strengthening, functional activities, and aerobic activity. In another study, Singh-Grewal et al. (32) randomized children to 12 weeks of vigorous land-based aerobic exercise or Qigong, a gentle relaxation programme similar to tai chi. The patients completed one supervised session a week and two sessions at home, using a video of the programme. Both groups showed significant and clinically important improvement in the CHAQ assessment, but no significant difference was found between the groups on the CHAQ or fitness outcomes, suggesting that high-intensity exercise did not confer any additional benefit. In a study by Takken et al. (16), patients were assigned randomly to an aquatic aerobic exercise regimen once a week for 20 weeks or to assessment only. The exercise group demonstrated no significant small- to medium-sized improvements in active joint count, function, or quality of life. Neither the VO2peak nor the 6-min walking distance changed in either group. Our results are consistent with the two previous randomized controlled studies that demonstrated significant benefits in functional and quality of life outcomes (31, 32). In terms of cost-effectiveness and accessibility, we considered our exercise programme more convenient than those used in the previous studies. Because a precise cure is not available for JIA, an exercise programme should be a feasible intervention to be administered by children’s parents or adolescents.
The strengths of our study are the randomized, controlled, single-blind design; the inclusion of power calculations; the use of valid outcome measures; and the involvement of a large sample size. Furthermore, our patients and assessors were blinded to the group allocation. However, there was some limitation in our study. Our rheumatology and physiotherapy department is one of the few high-quality reference centres in Turkey. Parents from many cities attend our centre in order to obtain a diagnosis for their children. Because of the transportation difficulty for more distant families, adherence to the programme decreases throughout the study. Another limitation is the use of exercise booklets to describe our exercise programme without video-based exercise education to demonstrate how to perform the exercise programme at home. Although we have controlled for compliance using a weekly diary, we cannot be sure about the quality of the exercise performance. This limitation may be a result of our individualized exercise programme.
In conclusion, this study demonstrated that an individually planned LBHE programme has the potential to improve functional ability and quality of life outcomes in patients with JIA. In addition to conventional medical care, we suggest that an exercise programme should be recommended to patients with JIA. Further research is needed to clarify outcomes among the diversity of possible exercise programmes with respect to the effects measured and to strengthen exercise management support. The long-term effect of exercise therapy remains unclear and requires further research.
ACKNOWLEDGEMENTS
The authors would like to thank to Turkan Ertugrul, MD, Prof.Dr. for contribution in study design and Burcu Ayhan, PT, MSc for contribution to communicate with patients in this study.
The authors declare no conflicts of interest.
REFERENCES
