Kiminobu Furukawa, PhD1, Harue Suzuki2 and Jun Fukuda, MD2
From the 1Faculty of Rehabilitation and Care, Seijoh University, Aichi and 2University of Human Arts and Sciences, Saitama, Japan
OBJECTIVE: To observe the real-time muscle activity of bilateral hands while subjects draw circles under 2 conditions: with and without using Ramachandran’s mirror-box.
SUBJECTS: A total of 24 healthy volunteers.
METHODS: Subjects drew 4 circles sequentially using their dominant hand with the other hand at rest, both with and without looking at a mirror image. Circles were marked by 8 dots on the paper, which subjects connected up to draw the shape. The activity of the bilateral first dorsal interosseus muscles was recorded using surface electromyography.
RESULTS: Muscle activity of the dominant hand remained constant during each task. In contrast, muscle activity of the non-dominant hand increased under the condition of watching the image in the mirror, but was low under the non-watching condition. Furthermore, muscle activity of the non-dominant hand increased over the duration of the task. However, wide variation between subjects was observed under the mirror-image condition.
CONCLUSION: Increased muscle action potential of the non-dominant hand may be induced by the circle drawing task of the dominant hand during Ramachandran’s mirror-box therapy, which supports previous observations of increased brain activity caused by watching a mirror image.
Key words: mirror-box; drawing; muscle; motor cortex; motor overflow; surface electromyography; functional laterality.
J Rehabil Med 2012; 00: 00–00
Guarantor’s address: Kiminobu Furukawa, Faculty of Rehabilitation and Care, Seijoh University, 2-172 Fukinodai, Tokai-city, Aichi 476-8588, Japan. E-mail: furukawa@seijoh-u.ac.jp
Submitted November 14, 2011; accepted June 19, 2012
Introduction
Ramachandran’s mirror-box therapy (1–4) is commonly used, with beneficial outcomes, to relieve phantom pain in amputees (5–7) and improve paralysis in stroke survivors (8–10). It is thought that a positive outcome may be due to changes in brain excitation induced when the patient watches a mirror image of their moving hand during therapy, as observed in previous studies (11–13). However, some recent studies and reviews regard this therapy with scepticism (14–17). Most previous studies have focused on changes in brain activity or on comparison of the pre- and post-intervention outcomes of this therapy; no studies have observed real-time changes in muscle activity during the therapy. When motor command from the human primary motor cortex is transferred to specific alpha motor neurones via the corticospinal tract, the muscle action potential of the target muscle occurs. In this study, surface electromyography (sEMG) was used to determine whether brain activity can induce muscle action potential during Ramachandran’s mirror-box therapy. If watching a mirror image does enhance muscle activity of the non-dominant hand during therapy, this may indicate that the change in brain activity induced by watching a mirror image may influence the motor function of the non-dominant hand.
Materials and Methods
Subjects
A total of 24 healthy volunteers (2 females, 22 males; hand dominance: 22 right, 2 left) were recruited to the study. Demographic data for the subjects are shown in Table I. Informed consent was obtained from all subjects prior to participation. The study was approved by the local ethics committee of Seijoh University (Aichi, Japan), and was performed in accordance with the 1964 Declaration of Helsinki.
|
Table I. Demographic data for the subjects |
|
|
|
Mean (SD) |
|
Age, years |
19.5 (1.1) |
|
Height, cm |
171.9 (5.6) |
|
Weight, kg |
61.9 (10.3) |
|
SD: standard deviation. |
|
Experimental procedure
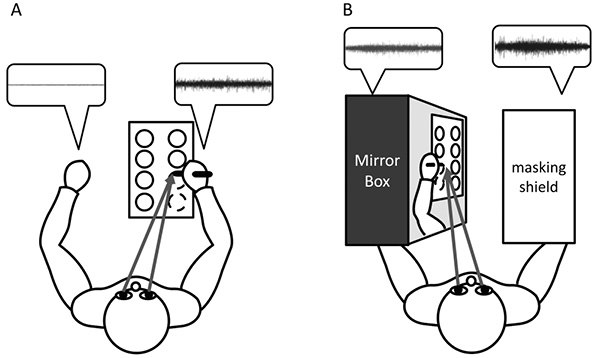
Fig. 1 shows the two experimental conditions of this study, and Fig. 2B shows the test paper used for the circle-drawing task. In most previous studies using the “drawing” task, subjects were asked to write their names or some words, but the present study used a circle-drawing task, because: (i) the shape looks the same when viewed in a mirror; (ii) subjects have to move their dominant hand skilfully in order to join the dots to make the circles. Four circles were aligned in pairs on an A4-sized test paper attached to a desk with adhesive tape (Fig. 2). Before the cue to start, subjects positioned their bilateral arm on the desk, at rest. Once subjects received the start cue, they began to draw 4 circles (C1, C2, C3 and C4) sequentially using the dominant hand. Each subject drew 4 circles under the conditions of watching (mirror-image condition) or not watching (non-mirror-image condition) a mirror image reflected in the mirror-box. The order of performing the tasks was randomized. In the pilot study, despite instructions to perform the task while watching the mirror image, some participants in the mirror-image condition occasionally performed the task while watching their dominant hand. To ensure task compliance, a masking shield was placed over the dominant hand. Subjects rested their non-dominant arm on the desk under the mirror-image condition, but inserted it into the mirror-box under the other condition. They kept the non-dominant arm at rest in either condition.
Fig. 1. Circle-drawing task, (A) with and (B) without watching a mirror image. Comparison of the raw surface electromyography (sEMG) waveforms (shown in the dialogue balloons) for the non-dominant hand revealed that the sEMG amplitude was high under the condition with watching a mirror image.
Surface electromyography
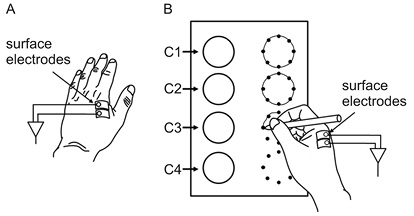
sEMG (TeleMyo G2, Noraxon Corp, Arizona, USA, sampling rate, 1,500 Hz, bandpass width, 10–950 Hz) was recorded from the first dorsal interosseus muscle of each hand. This muscle was chosen because it plays an important role in controlling the movement direction of a pen. Ag-AgCl surface electrodes (BlueSensor N-00-S, Ambu Corp, Copenhagen, Denmark) were attached to the centre of both subject muscle bellies at an inter-electrode distance of 2 cm (Fig. 2). Careful attention was given to skin preparation in order to set the skin impedance at less than 5 kΩ.
Observed sEMG signals were converted from analogue to digital and stored on a personal computer (Inspiron 5150, DELL Corp., Texas, USA) capable of synchronizing the image of a moving hand.
The drawing tasks require negligible output of the subject muscle. Thus, the observed raw sEMG waveform showed wide fluctuation. To resolve this problem, a moving mean method was applied at 30 ms windows to analyse the data (smoothing). Mean amplitude values and the incremental rate of amplitude change were calculated from the processed data.
Fig. 2. Surface electrode configurations and figure-drawing task. (A) Surface electrode location of the non-dominant hand. (B) Surface electrode location of the dominant hand and the test paper used for the circle-drawing task. The 4 circles on the left side are examples and those on the right are outlined with 8 dots for the patient to draw round.
Statistics
Two-factor repeated-measures analysis of variance (repeated-ANOVA) was used to analyse the temporal changes in muscle activity for each time period (C1, C2, C3 and C4) (period) and for each condition, with and without watching the mirror image (condition). In case of a significant difference, post-hoc analysis (Sheffe’s method) was performed. All statistical analyses were conducted using statistical software (StatView Ver. 5.0 for Windows, SAS Institute, North Carolina, USA), and the significance level was set at p < 0.05.
Results
Case study of a typical participant
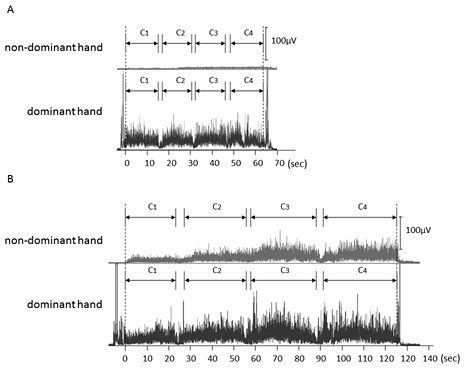
Fig. 3 shows the rectified sEMG waveforms observed for subject A, whose results are explicit examples for this study. Significant muscle activity was observed in the dominant hand during both tasks (Fig. 3A, B). In contrast, mean muscle activity of the non-dominant hand was approximately 5 µV when watching the mirror image, although a progressive increase in muscle activity was observed over the course of the task (Fig. 3A). However, muscle activity of the non-dominant hand when watching the mirror image was extremely high, and an apparent progressive increase was seen over the course of the task. The mean value of non-dominant hand muscle activity was approximately 20 µV, which was approximately 4 times higher than for the same hand under the non-mirror-image condition (Fig. 3B).
Fig. 3. Surface electromyography (sEMG) waveforms observed in subject A during the task (A) with and (B) without watching a mirror image. (A) sEMG waveforms of the non-dominant hand (upper panel) and dominant hand (lower panel) observed under the mirror-image condition. (B) sEMG waveforms of + non-dominant hand (upper panel) and dominant hand (lower panel) observed under the non-mirror-image condition.
Muscle activity of the dominant hand (Table II)
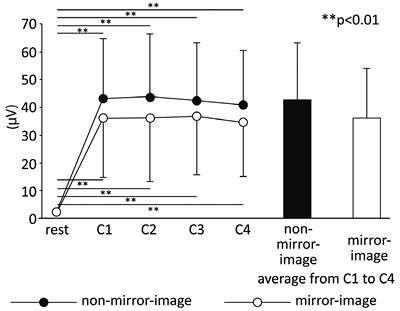
Fig. 4 shows the mean amplitude values of sEMG for the dominant hand for all subjects. In both conditions, muscle activity remained constant while the task was being performed. As soon as the task was started, a significant increase in muscle activity was evident (F(4, 184) = 111.09, p < 0.01) (period). Post-hoc analysis showed that muscle activity of the dominant hand was constant during the circle-drawing task. There were no significant effects of task condition (condition) and no interaction (condition × period) (F(1, 46) = 1.27, not significant (NS) and F(4, 184) = 0.85, NS, respectively).
Fig. 4. Mean surface electromyography amplitudes of the dominant hand.
|
Table II. Surface electromyography amplitude of the dominant hand during each task |
||||||
|
|
Rest Mean (SD) |
C1 Mean (SD) |
C2 Mean (SD) |
C3 Mean (SD) |
C4 Mean (SD) |
C1–C4 Mean (SD) |
|
Mirror-image condition (mV) |
2.1 (1.3) |
36.4 (22.8) |
36.2 (17.8) |
36.8 (18.5) |
34.8 (17.7) |
36.0 (17.9) |
|
Non-mirror-image condition (mV) |
2.1 (1.1) |
43.2 (21.6) |
43.4 (23.0) |
42.2 (21.0) |
40.5 (19.8) |
42.3 (20.5) |
|
SD: standard deviation. |
||||||
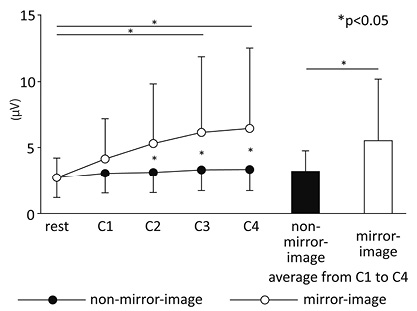
Muscle activity of the non-dominant hand (Table III)
Fig. 5 shows the mean amplitude values of sEMG for the non-dominant hand of all subjects. While there was no difference in muscle activity during the rest period, higher muscle activity (F(1, 46) = 4.54, p < 0.05) (condition) and its temporal increase (F(4, 184) = 9.28, p < 0.01) (period) were observed under the mirror-image condition. Moreover, a significant interaction of the two factors (condition × period) was seen (F(4, 184) = 5.24, p < 0.01). Post-hoc analysis showed that the muscle activity of the non-dominant hand increased progressively when watching the mirror image (rest–C2, C1–C4, p < 0.05; rest–C3, rest–C4, p < 0.01). This result was not observed when not watching the mirror image. Furthermore, muscle activity observed at each period during the task was higher when watching the mirror image than when not watching it.
Fig. 5. Mean surface electromyography amplitudes of the non-dominant hand.
|
Table III. Surface electromyography amplitude of non-dominant hand during each task |
||||||
|
|
Rest Mean (SD) |
C1 Mean (SD) |
C2 Mean (SD) |
C3 Mean (SD) |
C4 Mean (SD) |
C1–C4 Mean (SD) |
|
Mirror-image condition (mV) |
2.6 (1.6) |
4.1 (3.1) |
5.3 (4.5) |
6.1 (5.8) |
6.4 (6.1) |
5.5 (4.7) |
|
Non-mirror-image condition (mV) |
2.8 (1.6) |
3.0 (1.5) |
3.1 (1.5) |
3.3 (1.6) |
3.3 (1.6) |
3.2 (1.6) |
|
SD: standard deviation. |
||||||
Drawing speed
Drawing speed during the task performed when watching the mirror image was slower than for the task performed without watching it (condition) (F(1, 46) = 58.53, p < 0.01). Furthermore, a gradual increase in drawing speed was observed as the task progressed when the task was performed without watching the mirror image (period) (F(3,138) = 5.56, p < 0.01) (C1–C2, C1–C3, C1–C4; p < 0.01), but not when the task was performed when watching it. There was no significant interaction between the two factors (condition × period) (F(3,138) = 0.44, NS).
Discussion
In this study 24 healthy subjects drew 4 circles sequentially with their dominant hand. An increase in muscle activity of the opposite hand that was at rest behind the mirror was measured. This phenomenon was not observed if the task was performed when not watching the mirror image. To the best of our knowledge, this is the first study to assess muscle activity during a task involving the use of Ramachandran’s mirror-box. Non-dominant hand muscle activity observed during the task when watching the mirror image was 2–6 times higher than that measured in the control task. Although the variation in this difference was large, this phenomenon was found in most of the subjects.
Moreover, by analysing the temporal changes in muscle activity of the non-dominant hand during the task under the mirror-image condition, the following observations were made: (i) onset of muscle activity was late for the start of the circle-drawing task; (i) muscle activity increased with task progress; (iii) muscle activity decreased to a level equivalent to that of the rest period once the task was finished; and (iv) muscle activity was not affected by taking up or putting down the pen.
Muscle activity defined as unintentional or unnecessary activity accompanying voluntary movement, has been called “motor overflow” (18, 19). Previous studies have reported the following characteristics for motor overflow: (i) large muscle activity induces a large motor overflow of contralateral homonymous muscle; (ii) motor overflow increases under fatigue; (iii) older people have larger motor overflow than young people; and (iv) motor overflow is increased by performing a dual task. Although the exact mechanism underlying the production of motor overflow is unclear, two theories have been described in previous studies. One is that brain excitation spreads from the active motor area to the contralateral passive motor area through the callosum (20). The other concerns brain excitation transmission through the ipsilateral corticospinal tract (20). Shinoura et al. (13) reported that the primary motor cortex (M1) of the affected hemisphere in stroke survivors was activated by mirror-box therapy. Their finding suggests that the observation of a mirror image induces significant brain excitation of the contralateral hemisphere. Nojima et al. (11) demonstrated that mirror visual feedback improved excitatory connections of M1 by using transcranial magnetic stimulation. While these studies correlated the beneficial effect of mirror-box therapy with activity of contralateral primary motor cortex, Michielsen et al. (12) reported an increase in activity in the precuneus and the posterior cingulate cortex, but no M1 activity. The results of this study suggest that mirror illusion may induce unintentional M1 activity according to the two theories of motor overflow.
There was a large inter-subject difference in muscle activity observed in this study. Though a statistical difference was found when comparing the two conditions (mirror-image vs. non-mirror-image), the muscle action potential was unexpectedly small. If mirror illusion modifies the motor overflow response, the results observed in this study may rest on the following factors: (i) since the circle-drawing task required little effort for all subjects, the induced motor overflow was small; (ii) all subjects in the study were young; (iii) target muscle was activated in various patterns, because each subject grasped the pen differently; and (iv) it is possible that different recognitions of the mirror image by each subject influenced the motor overflow response. Factors (ii) and (iii) may produce a large inter-subject difference; hence, it is speculated that most previous research reports of clinical trials focused on the effect of mirror-box therapy were case reports.
In conclusion, the results of this study suggest that muscle action potential in a non-dominant hand may be induced by a circle-drawing task of the dominant hand during Ramachandran’s mirror-box therapy. Furthermore, this muscle action potential can be detected in real time using sEMG. However, we could not pursue the factor of large inter-subject difference of muscle action potential of the non-dominant hand during circle-drawing tasks under the mirror-image conditions in our study. Although sEMG detects brain excitation indirectly, a synchronized system with equipment that measures brain excitation directly may be able to explain the relationship between excitation in the central nervous system and peripheral muscle activity during this therapy. In addition, the results of this study may not apply to people of all ages, since our subjects were all healthy young adults. Thus, these aspects of Ramachandran’s mirror-box therapy require further study.
Acknowledgement
This study was supported in part by a Seijoh University grant.
References
