OBJECTIVE: Lack of sensory feedback is a drawback in today’s hand prostheses. We present here a non-invasive simple sensory feedback system, which provides the user of a prosthetic hand with sensory feedback on the arm stump. It is mediated by air in a closed loop system connecting silicone pads on the prosthetic hand with pads on the amputation stump. The silicone pads in a “tactile display” on the amputation stump expand when their corresponding sensor-bulb in the prosthesis is touched, evoking an experience of “real touch”.
METHODS: Twelve trans-radial amputees and 20 healthy non-amputees participated in the study. We investigated the capacity of the system to mediate detection of touch, discrimination between different levels of pressure and, on the amputees also, the ability to locate touch.
RESULTS: The results showed a median touch threshold of 80 and 60 g in amputees and non-amputees, respectively, and 90% and 80% correct answers, respectively, in discrimination between 2 levels of pressure. The amputees located touch (3 sites) correctly in 96% of trials.
CONCLUSION: This simple sensory feedback system has the potential to restore sensory feedback in hand amputees and thus it could be a useful tool to enhance prosthesis use.
Key words: sensory feedback; prosthesis; forearm amputees.
J Rehabil Med 2012; 44: 702–707
Correspondence address: Birgitta Rosen, Department of Hand Surgery, Skåne University Hospital, SE-205 02 Malmö, Sweden. E-mail: birgitta.rosen@med.lu.se
Submitted September 22, 2011; accepted February 28, 2012
Introduction
A major goal in applied neuroscience is to create artificial limb devices that feel and act like real limbs, and provide an intuitive use requiring less visual attention (1–3). To achieve this goal prostheses must have sensation, i.e. tactile feedback. There are no commercially available sensory feedback systems for artificial limbs today. Various approaches of sensory feedback systems have been presented over the years, but no proposed system has yet been convincingly proven usable (4–12).
The process of providing sensory feedback is 3-fold: (i) registration of tactile stimuli by an artificial receptor organ, i.e. sensors; (ii) actuators to transfer the tactile stimuli from the artificial receptors to intact skin; (iii) a relearning with adaptation of the central nervous system to the new type of afferent signals. Without this third step it can never be a true sensory feedback.
The aim is to offer prosthetic users a prosthesis system providing a perception of touch as close as possible to physiologically natural perceived sensation. This implies a sensory feedback system that goes beyond an intrinsic loop between sensors and motors in a prosthesis, which, for example, detects if an object is slipping and automatically adjusts the grip force accordingly (13) without the awareness of the user. The goal must be a true perception that provides a conscious sensibility that results in a feeling of body-ownership of the prosthesis (4, 14–16). The technical solution should preferably be non-invasive, simple, durable, and not interfere negatively with the myoelectric or cosmetic functions of the prosthesis.
Within the SmartHand project (EU grant number NMP4-CT-2006-00334231) we have published a concept of transferring sensation from a prosthetic hand onto the remaining forearm of an amputee to create sensory feedback (17–19). Most amputees experience phantom limb sensations and/or phantom limb pain, as well as residual limb stump pain. There is often a “map” of the phantom hand on the amputation stump, where pressure on specific skin areas results in an evoked sensation from specific fingers in the amputated hand (15, 20, 21). Phantom phenomena are not homogeneous; each patient presents with a unique combination of spontaneous or evoked sensations, pain, and/or awareness (20). Functional magnetic resonance imaging (fMRI) results have shown that the somatotopy of such phantom hands in amputees, following tactile stimulation of the distal hand map in the amputation stump, corresponded topographically with that of a normal hand (22). This skin area, the hand map distal in the amputation stump, serves as a potential target for sensory feedback.
Historically, experimental models are described that use electrotactile feedback, where a small electric current is passed through the skin (23, 24), or with vibrotactile feedback (25, 26), which is the most common medium used. Thermal feedback has also been proposed (27). Our own group has discussed the possibilities of using another sense, hearing, using a glove equipped with microphones, for sensory feedback (28). Pylatiuk et al. (26) report on a vibrotactile feedback system tested on 5 persons with left forearm amputation. Furthermore, Marasco et al. (4) report a mild tingling or shocking sensation in the target skin tested on 2 subjects who had undergone targeted reinnervation surgery.
We have previously described that a sense of body ownership of a prosthetic device is possible after simultaneous stimulation of the prosthesis and the stump (15, 16). This is a process that involves multisensory areas of the brain, tricking the brain into experiencing sensation of touch from the artificial finger (28). If the prosthesis is experienced as being a part of the body in this way, it is probably easier and more intuitive to use.
We introduce here a new approach (to an old idea) on how to mediate sensory feedback from a prosthetic limb. In this device, a tactile stimulation is delivered instantaneously to the stump hand map every time the finger of the prosthesis touches an object, thereby inducing the brain into experiencing the sensation of touch from the artificial hand (29–31). This method also uses the capacity of the brain to adopt in response to external changes and it provides a relatively easy way to restore rudimentary tactile sensibility in a prosthesis (22, 32, 33).
It is a simple one-to-one passive sensory feedback system based on a closed-loop control that purposely makes use of exteroceptive as well as cognitive qualities. Such a close-loop system to achieve sensory feedback in prostheses was patented by Rosset in 1933 “finger pressure was transmitted directly to the residual limb by mechanical or pneumatic means so that finger pressure could be directly related to pressure on the residual limb” (6). Our design has taken the old simplistic feedback idea a step further and provides the user with sensory feedback on the trigger points of the “map” of the phantom hand that can be defined in the stump in most amputees (20). The sensory feedback is mediated via a non-invasive closed pneumatic system of silicone encapsulated bulbs. The bulbs in the prosthesis are connected to silicone pads, formatting a “tactile display” on the arm stump, and the feedback that this pneumatic system gives at touch is very close to the experience of real touch.
The technical solution for the sensory feedback system is presented here. An initial investigation was undertaken in amputees and non-amputees to monitor the capacity of the sensory feedback system to mediate tactile input with regards to touch threshold, capacity to differentiate between different levels of touch and the capacity to localize touch. Results from a single amputee testing a prosthesis equipped with the sensory feedback system are also presented.
MethodS
Sensors to pick up tactile stimulation and actuators for generating the tactile sensation
Silicone bulbs to pick up tactile stimulation were fabricated using high-temperature vulcanized (HTV) silicone in the range of 20–65 shore. The bulbs had a grip-membrane of a 0.5-mm thin 20 shore silicone and 1.75 mm 65 shore as a bottom-support to form a semi-rigid wall. The bulbs were attached to a rigid plastic rod, forming the prototype fingers. Actuators forming a “tactile display” to be placed on the forearm were designed using silicone pads fabricated using HTV-silicone. The pads had a membrane of 0.3-mm 20 shore silicon with a 35 shore back support covered by a rigid thermoplastic socket in order to make the silicone bulge only in the direction of the skin. The side facing the skin was made as thin as possible to allow bulging against the skin and minimize counterforce due to a thick membrane. The pads had a membrane-diameter of 10 mm and a total diameter of 20 mm. The volume of the sensor bulbs is approximately 5 ml and the volume of the actuator pad is 2 ml. A recording of the pressure from the sensor pads using a MPX5050DP sensor (Freescale Semiconductor, Austin, Texas, USA) was performed and using monofilaments to apply the pressure. A simulation of the membrane of the actuating pad was performed in COMSOL (COMSOL AB, Stockholm, Sweden) and the maximum deflection of the membrane was computed using the pressures measured when using the monofilaments (Table I).
| Table I. Measured pressure from the sensing pads when using monofilaments and the corresponding simulated deflection of the actuator pads |
| Filaments used, g | Measured pressure, kPa | Simulated deflection, mm |
| 60 | 1.2 | 0.9 |
| 100 | 2.3 | 1.7 |
| 180 | 4.3 | 2.8 |
| 300 | 6.5 | 3.9 |
A tactile display with 3 corresponding silicone pads in 3 separate closed loops was used in the experiments. Plastic tubing was used to connect the bulbs and the pads (PUN-3 × 0.5 SI, FESTO, Esslingen am Neckar, Germany).
Experimental set-up
Thirty-two participants, 12 amputees (Table II) and 20 non-amputees (10 males and 10 females, mean age 41 years) took part in the experiments. All participants gave their consent and the experiments were conducted in accordance with the Declaration of Helsinki. The local ethics committee of Lund University approved the study. The experiments were designed to establish:
• touch threshold in the system
• capacity to differentiate between different levels of pressure
• capacity to localize touch.
| Table II. Details of participating amputees |
| Gender/ age, years | Phantom map in the stump | Cause of amputation | Time since amputation, years | Prosthesis |
| M/42 | Yes | Traumatic | 27 | Myoelectric |
| M/47 | No | Congenital | – | Cosmetic/aesthetic |
| M/39 | Yes | Traumatic | 26 | Cosmetic/aesthetic |
| M/76 | No | Traumatic | 65 | Cosmetic/aesthetic |
| M/66 | Yes | Traumatic | 47 | Cosmetic/aesthetic |
| M/23 | Yes | Traumatic | 1 | Myoelectric |
| M/40 | Yes | Traumatic | 27 | Cosmetic/aesthetic |
| M/58 | No | Traumatic | 11 | Myoelectric |
| M/58 | No | Traumatic | 11 | Myoelectric |
| F/35 | No | Congenital | – | Cosmetic/aesthetic |
| F/70 | No | Congenital | – | Cosmetic/aesthetic |
| F/38 | No | Congenital | – | Myoelectric |
| M: male; F: female. |
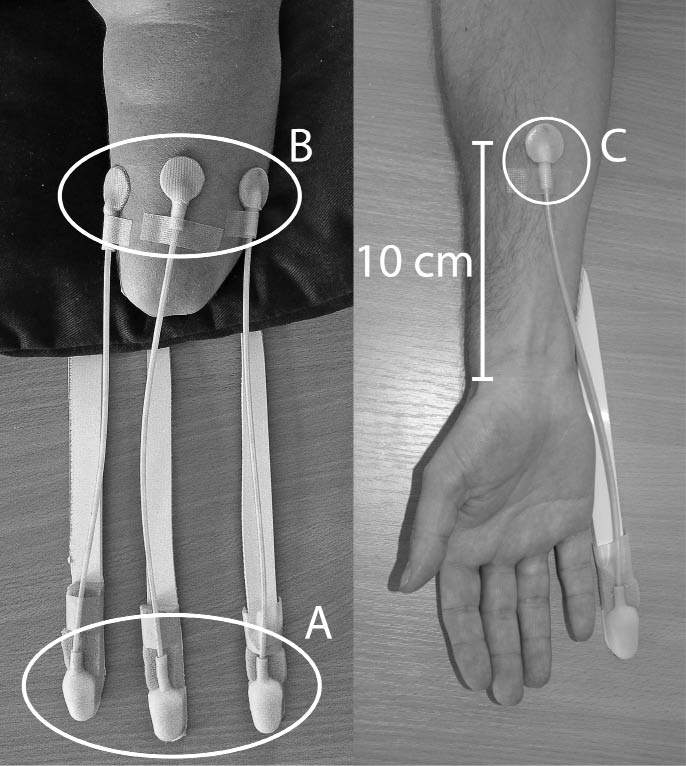
The test participant was seated comfortably with the arm tested on the table in a resting position and with their vision occluded. The silicone bulbs (sensors, Fig. 1A) were positioned on the table and the silicone pads (actuators, Fig. 1B) constituting the tactile display were positioned on the amputation stump of the amputees. To find out the location of the zones of the referred sensations (the phantom hand map) the participant was asked to touch on the stump, and define the referred phantom parts of the hand (digits I–V). The zones on the stump were then marked with a pen, and then the participant verified the mapping by touching the marks. The participant then chose 3 distinct zones to use during the experiment. In subjects with no present phantom hand map 3 random zones on the stump with clear sensory function were chosen. In the non-amputees the forearm was used to attach the silicone pad. A single zone on the mid-volar aspect 10 cm proximal to the wrist was used as test-zone (Fig. 1C).
Fig. 1. Experimental set-up for the amputees (left) and non-amputees (right). (A) Silicone bulbs. (B) Silicone pads on the residual limb of an amputee. (C) Position of the silicone pad on non-amputees.
Experimental procedure
Amputees. Three different experiments were performed consecutively and in the same order in each test subject. The first experiment included identification of touch threshold for perception of touch/pressure from a single sensor bulb. For this experiment a full set of 20 Semmes-Weinstein monofilament (SWM) was used (North Coast Medical, Gilroy, CA, USA). The Semmes–Weinstein monofilaments exert a force ranging from 0.008 to 300 g when bowed into a C-shape against the skin for 1 s (34). While “normal” touch threshold in uninjured normal hands is in the lower end (≤ 0.07 g) of this ordinal scaled instrument, the touch threshold obtained in the present study was in the upper end among the 5 heaviest monofilaments (26, 60, 100, 180, 300 g). Filaments are calibrated to provide a specified force measured in grams. The force delivered to the skin surface is the force divided by the surface area of the filament (35). This is a standardized instrument for screening/establishing level of touch thresholds used in hand rehabilitation (34). During the tests the subjects wore an eye-mask, the tested arm was comfortably resting, and the equipment with silicone bulbs was connected to the silicone pads on the forearm. The test subject was instructed to indicate when they felt the touch on the forearm. Pilot tests had indicated that touch threshold for the system were far above “normal” (70 mg), thus assessment was started with SWM #4.31 (2 g), and thereafter in an ascending or descending order depending on the answer to the first filament. This was repeated 10 times in 1 of the 3 zones on the stump/forearm. Each filament was applied 3 times according to standardized procedure (34). The second experiment included identification of two levels of pressure from a single sensor bulb. Two supra-threshold monofilaments (180 and 300 g) were used for the discrimination task in the second experiment. They were randomly applied 30 times on the silicone bulb and the number of correct answers (high/low) was noted.
The third experiment included identification of the location of finger corresponding to a stimulus from 3 sensor bulbs. The 3 zones on the forearm were connected to silicone bulbs on the table, the test subject was blindfolded and the 3 silicone bulbs were then randomly touched with a supra-threshold touch 30 times. The test subject indicated location (e.g. thumb, second or fifth digit).
Non-amputees. In the non-amputees the third experiment, identification of the location of finger corresponding to a stimulus from 3 digits, was not performed. However, identification of touch threshold for perception of touch/pressure, and identification of two levels of pressure from a single digit was performed in a similar way as on the amputees.
Analysis
A group comparison was performed using the Mann-Whitney test in SPSS.
Results
The median touch threshold mediated by the sensory feedback system did not differ significantly between the two groups (p = 0.18), 80 g (interquartile range (IQR) 60–100 g) in the amputee group, and 60 g (IQR 60–100 g) in the non-amputee group. However, the capacity to discriminate between two levels of pressure was significantly better in the amputee group (p < 0.05) with 90% correct answers (range 55–100) compared with 80% correct answers in the non-amputee group (range 43–100).
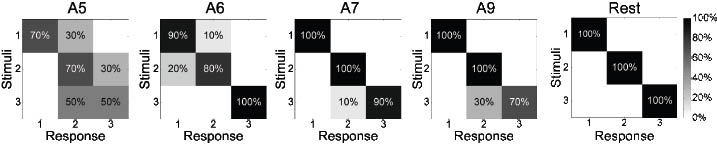
The capacity to identify the location of finger corresponding to a stimulus from 3 digits was 96% correct answers in the amputee group (range 63–100). Confusion matrices of this test can be seen in Fig. 2. Eight amputees reached a perfect score and 3 amputees had accuracies above 90%.
Fig. 2. Confusion matrices for identification of the location test. Amputees (A) 5, 6, 7 and 9 did not get a perfect score, whereas all the rest did.
A field-test of the sensory feedback prototype
A myoelectric prostheses (Otto Bock MyoHand VariPlus Speed, size 7¼) (36) was provided with the aforementioned sensory feedback (Fig 3A). The bulbs were integrated in a silicon prototype glove using HTV silicone in the range 20–65 shore. The glove was made one size larger than the electric hand (7¾ glove for a 7¼ hand). The bulbs had a grip-membrane of a 0.5mm thin 20 shore silicone and 1.75 mm 65 shore as a bottom-support to give a semi-rigid wall to transfer the grip force from the electric hand. The bulbs were made in different sizes depending on the area each finger allowed.
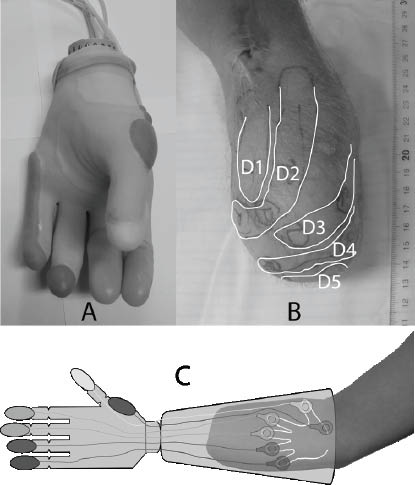
Fig. 3. (A) Otto Bock MyoHand VariPlus Speed fitted with the sensory feedback system. (B) Phantom finger mapping of the amputee who took part in the study. (C) Conceptual illustration of the whole system. Sensing bulbs on the prosthetic fingers to the left and actuating pads on the “phantom fingers” to the right.
Based on monitoring of patients using their prosthesis it was equipped with 6 silicone bulbs positioned on the tip of the thumb, index, third and fifth finger. The two remaining bulbs were placed at the most radial and ulnar position of the prosthesis.
This “naked” prototype was field-tested by a patient with 25 years of experience with full-time use of myoelectric hand prosthesis. He had a very distinct and detailed phantom hand map on his stump (Fig. 3B) and it was easy to find zones on the stump that corresponded well with the ones on the prosthesis. A conceptual description of the sensory feedback system can be seen in Fig. 3C.
After two weeks use of the prosthesis at home and at work, a few of his comments were: “After almost 30 years with a prosthesis without sensibility I put this on and get a feedback when I touch things – and it just works! I can even trust that I hold something without looking”, “The lab-tests felt good, but using it in my daily activities gave me a wow- experience”, “The silicone bulbs in the fingertips give a soft grip that makes it easier to hold fragile objects like a plastic cup of water”, “Some of the feedback zones on the stump disappeared in the muscle-noise when activating the motor-function in the prosthesis during, e.g. lifting things, and in that situation there is also a clear mechanical feedback from the edge of the socket against the stump”, and “Occasionally there was some leakage of the air in the system”.
Discussion
We present here a simple non-invasive sensory feedback system that provides the user with a sensory feedback on the amputation stump via a closed-loop passive pneumatic system. The touch threshold on the prosthesis is substantially higher than in a normal hand, i.e. it is a rough feedback in terms of quantity of force required on the prosthesis. However, an important aspect is that the feedback is mediated to the phantom map of the hand and thus easily triggers the hand area in the somatosensory cortex. This, in combination with a modality matched feedback, enhances the quality of the feedback to be very close to true touch and it has a potential towards embodiment of the prosthesis.
In our previous studies that describe a concept for sensory feedback in prosthetic limbs we used computer-generated stimuli (18, 19). In the present study we have moved one step forward towards clinical applicability with a direct physical connection between the stimuli and the forearm skin, where the stimuli is detected and mediated into a conscious touch. One of the amputees that participated in this study also used a prototype system embedded in a standard myoelectric prosthesis in a field-test during a couple of weeks.
Marasco and co-workers (4) have described, in two amputation cases with targeted reinnervation, how a neural-machine-interface that provides a physiologically appropriate artificial sense of cutaneous touch seems to elicit a shift in perception towards incorporation of a prosthetic limb into the self-image, and thus provide the possibility that a prosthesis becomes not only a tool, but also an integrated body part.
In light of the 3-fold process of providing sensory feedback in prosthetic limbs, our new approach to the old idea, patented by Rosset in the 1930s, focusses not only on sensors and actuators on the prosthesis and the intact skin, but also on the cognitive aspect.
The presented system makes use of the stump hand map seen in most forearm amputees. Neuro-imaging studies have shown that stimulation of the hand map in the stump results in activation of the corresponding finger areas in the primary somatosensory cortex (22). Using the map areas in the stump for sensory feedback instead of any stump area has the advantage of not requiring a learning phase where the amputee has to learn to associate pressure at specific points in the stump with pressure on different fingers in the prosthesis. Interestingly, the amputees in this study performed better in the discriminative task than the non-amputees. This may indicate a capacity to change the perceptive qualities in the forearm skin. The stump hand map varies a great deal between amputees; some have a very detailed map of several fingers and others have a “simpler map” or no map at all (as in 6 of our cases). In these latter cases the described system can be used, but the actuators on the stump have to be placed on “random” skin areas, requiring a learning process based on brain plasticity, which takes time. Furthermore, this process is also subjected to large inter-individual differences based on different individual cognitive capacities. The 6 amputees in our study, subject 8 and 9 is a bilateral amputee, with no “hand map” on either side performed equally well in the discriminative tasks, as did the ones with a detailed “hand map”. This learning process and especially how to improve the accuracy in the learning, i.e. inducing a hand map in the amputation stump, has to be addressed in further studies.
Furthermore, the better the sensory feedback the more likely it is for the prosthesis to be incorporated in the body schema, i.e. induction of body ownership.
Childress discussed in a review that in existing prosthetic devices loops are closed by the user through vision and incidental stimulation from socket pressure and sound from the motor, etc. (6). This was the case in 1980, and not much has changed. Our design has taken the old simplistic feedback idea a step further and provides the user with a sensory feedback on the trigger points of the “map” of the phantom hand, which can be defined in the stump in most amputees, mediated via a closed pneumatic system of silicone encapsulated bulbs.
To our knowledge there is no commercially available system for true sensory feedback in prosthetic devices, apart from intelligent prostheses with slip-sensors in the fingers that provide feedback to the grip strength-adjuster in the prosthesis. Previously described systems have mostly used sensory substitution, meaning that there is a modality mismatch, i.e. pressure or force recorded on the fingertips of a prosthetic hand is not only transferred to the residual limb but also transformed in the process.
The proposed sensory feedback system provides a solution that has a one-to-one modality matching, as well as, when using the stump hand map as the target for transfer of the stimuli, a one-to-one somatotopic matching. We consider both aspects to be of importance for providing a physiologically relevant feedback.
Prosthetic weight matters, and a part of the concept for this technical solution is a robust and simple equipment that can easily be fitted in “any” prosthesis. The material in the system is silicone and plastic, and a kit with 6 sensors (sensors, plastic tubes and actuators) and the added weight to the whole prosthetic system is negligible. Today the system is based on a closed passive pneumatic system and the force needed on the air-filled sensor-bulbs in the prosthesis to evoke a perception of touch in the other end of the system (the median trigger point of the phantom hand map on the stump) was 80 g in the amputees and 60 g in the non-amputees, with a range of 26–180 g. Hence the threshold to exert a conscious sensibility in this system is far from “normal” sensibility. This is probably a price for a simple and robust system that has to be valued in the light of what it can add to the user from a functional point of view. The patient in this study who used the system in his prosthesis for a couple of weeks also pointed out that the feedback was “quite subdued and demands some concentration”.
There are, of course, technical solutions for magnifying the pressure-evoked signal in the prosthetic fingers, thereby lowering the touch threshold, but that would take a much more advanced energy-consuming, heavy, fragile and probably slower technology. The main technical problem with the system presented here is leakage of air. The pneumatic loop must be air-tight and must not break when the prosthesis is used in normal daily activities.
A major principle in our system is to use the “mapping” of the phantom hand and the trigger points that can be defined in the stump for placement of the actuators. Most amputees experience this phantom limb sensation, but the phenomenon is not homogeneous (33). Often the trigger-points that evoke a feeling of touch in phantom digits are situated close together far distally in the stump. This can, of course, pose a practical problem.
Size matters too; the silicone pads (actuators) have a 10 mm2 effective pneumatic area. During the developmental process we have experimented with pads of various sizes, but there was no difference in touch thresholds between this small one and larger ones. The length and configuration of amputation stumps are individual to a high degree, as is the quality of the skin (20). In the socket of a myoelectric prosthesis the placement of the electrodes for control of the prosthesis must also be considered. The field-test in this study indicated that, when an actuator is too close to one of the electrodes, the pure movement of the muscle can make the perception of sensory feedback vanish.
A key question is where in the prosthesis the sensors should be located. The spontaneous answer is, of course, in the finger pads. However, monitoring how amputees handle their prosthesis in daily activities, and which parts of the prosthesis are in contact with the environment, reveals that, apart from the grip areas in the digits, the outer parts of the prosthesis, i.e. the most radial, ulnar and the very distal parts of the prosthesis, are often used to stabilize action. This is, of course, very much dependent on the type of prosthesis used (active or passive), and prostheses are still far from equivalent to real hands, so this is definitely a question that should be subjected to further investigation. In our hypothesized future sensory feedback “kit”, applicable to different kinds of prostheses, the placement of sensors as well as actuators should be individual.
In further development of the system continuous communication with potential users is of outmost importance, e.g. when it comes to the optimal placement of sensors. We can, however, already see several advantages with the presented system that might have positive effects, not only for users of myoelectric hand prosthesis, but possibly also for users of cosmetic/aesthetic prosthesis in the upper limb.
Acknowledgements
This study was supported by the Stockholm Brain Institute, the Swedish Research Council, the Crafoord Foundation, the Promobilia Foundation and Skåne County Council Research and Development Foundation.
References