Francesco Riganello, PhD1, Giuliano Dolce, MD1 and Walter G Sannita, MD2,3
From the 1S. Anna Institute and RAN – Research in Advanced Neurorehabilitation, Crotone, 2Department of Neuroscience, Ophthalmology and Genetics, University of Genova, Genova, Italy and 3Department of Psychiatry, State University of New York, Stony Brook, NY, USA
OBJECTIVE: To review the applicability of heart rate variability measures in research on severe disorder of consciousness.
METHODS: The available evidence on the correlation between heart rate variability measures and the outcome or residual functional state/responsiveness of severely brain-injured patients (including those in vegetative or minimally conscious states) are reviewed and discussed with reference to the central autonomic network model.
RESULTS AND CONCLUSION: Heart rate variability analyses appear to be applicable to assess residual or emerging (higher level) function in brain-injured patients with disordered consciousness and to predict outcome. In this regard, the central autonomic network model is heuristic in the understanding of heart rate variability descriptors of the central nervous system/autonomic systems relationship.
Key words: disorder of consciousness; brain injury; heart rate variability; HRV; vegetative state; minimally conscious state; central autonomic network.
J Rehabil Med 2012; 44: 495–501
Correspondence address: Giuliano Dolce, S. Anna Institute and RAN – Research in Advanced Neurorehabilitation, Crotone, Italy. E-mail: giulianodolce@libero.it
Submitted September 29, 2011; accepted February 16, 2012
INTRODUCTION
Subjects in a vegetative state (VS; today also referred to as unresponsive wakefulness syndrome) after severe brain injury are, by definition, disconnected from the environment, with no indication of awareness, voluntary or otherwise, purposeful movement, or communication (1–5). Autonomic functions are thought to prevail on central nervous system activities. In contrast, research by advanced positron emission tomography (PET) or functional magnetic resonance imaging (fMRI) techniques has documented stimulus- or condition-related regional brain activation that reflects retained connectivity in segregated networks. These observations are deemed indicative of surviving sensory, emotional and “cognitive” modular processing at varying levels of functional complexity in the absence of the integrative processes necessary to consciousness (5–14). The clinical scenario and perspective have expanded significantly, with far-reaching implications and requirements as to healthcare and neurorehabilitation of subjects in the VS. Emerging evidence suggests that the autonomic system can also mediate in patterns of brain activation at varying levels of complexity, and measures of heart rate variability (HRV) are applicable in the description of the brain functional organization in homeostasis and homeostatic response (15–18).
METHODS
The US National Library of Medicine Database and Google Scholar databases were used to trace published reports on HRV, VS, minimally conscious state (MCS), and autonomic system/function over the period 1993–2011, using appropriate keywords and their combinations. Cohort studies, case control studies, case reports and case series of adult or paediatric brain-injured patients were included in this review. Animal studies were not included.
HEART RATE VARIABILITY: MEASURES AND MEASUREMENTS
Measures of the HRV reportedly indicate or anticipate cardiac disorders (19–21) and reflect the action of physiological factors modulating the heart rhythm and its adaptation to changing conditions. The dynamic interplay between the autonomic subsystems enables efficient cardiovascular responses to endogenous/exogenous influences (22–24) and the efficiency of these responses can be quantified by appropriate data processing.
HRV recording techniques are non-invasive and HRV signals (the heart tachogram, i.e. the variation over time of the interval between consecutive heartbeats) have excellent signal-to-noise ratio compared with most brain signals in use in neuroscience or clinical neurophysiology, but are not periodic. Stimulus- or condition-related changes occur within the heart rate physiological range of variability in the absence of cardiac disorders and are seldom detectable without appropriate data treatment. To this purpose, the tachogram needs processing in the time or frequency domains or by geometrical or non-linear methods, as suggested by the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (25, 26). HRV fluctuations are conventionally measured in the time domain by calculating indices based on statistical operations on RR intervals; fast Fourier transform (FFT) or autoregressive models (26) are of common use in analyses of frequency. The HRV spectral profile is characterized by 3 main components: the high-frequency interval (0.15–0.5 Hz; HF), mainly associated with activation of the parasympathetic nervous system; the low-frequency interval (0.04–0.15 Hz; LF), reflecting contributions from both the parasympathetic and sympathetic systems; and the very-low-frequency bandwidth (< 0.04 Hz; VLF), thought to reflect temperature, vasomotor, hormonal, and metabolic regulation. The LF/HF ratio is typically used as a measure of the sympathovagal balance.
HRV descriptors are also derived by non-linear methods, such as entropy analysis, in order to describe the complexity, irregularity or randomness of HRV and its changes (27–30). Developments in the non-linear analysis theories provide new instruments of the data analysis in the entropy domain, such as the approximate entropy (ApEn) and the simple entropy (SapEn), which are thought to provide global information on autonomic system functioning and complexity (Table I).
|
Table I. Heart rate variability (HRV) measures
|
|
HRV analyses
|
Description
|
Output variables
|
|
Time domain
|
Statistical processing of consecutive intervals
|
HR, SDHR, NN, SDNN, RMSDD SDNN, pNN50
|
|
Frequency distribution
|
TINN (baseline width of the RR interval histogram), HRV triangular index (integral of the RR interval histogram divided by the height of the histogram)
|
|
Frequency domain
|
Frequency spectrum
|
FFT and AutoRegressive Analysis
Power: Total, ULF (< 0.003 Hz), VLF (0.003–0.04 Hz), LF (0.04–0.15 Hz), HF (0.15–0.4 Hz), Normalized Unit (LF, HF)
Time spectrum analysis
|
|
Non-linear analyses
|
Detrended fluctuation analysis (measures the correlation within the signal)
|
Typically the correlations are divided into short-term (α1) and long-term (α2) fluctuations
|
|
Poincare plot (graphical representation of the correlation between successive RR intervals)
|
SD1 (short-term variability)
SD2 (long-term variability)
|
|
Entropy
|
Measures of the complexity or irregularity of the signal (ApEn, SampEn)
|
|
ApEn: approximate entropy; SampEn: sample entropy; pNN50: proportion greater than 50 ms; RMSDD: root mean square of standard deviation; SD: standard deviation; SDNN: standard deviation of 5 min means; HF: high frequency; ULF: ultralow frequency; VLF: very low frequency; LF: low frequency; FFT: fast Fourier transform; HR: heart rate.
|
HRV measures are now being regarded with increasing interest as reliable descriptors of autonomic reaction to events with emotional resonance, and there is evidence that HRV can reflect the CNS/autonomic functional interaction under conditions involving motor, cognitive, emotional, behavioural or stressful tasks or adaptation to environmental change (16, 27, 31, 32). Clinical application is mainly in the investigation of subjects with psychiatric disorders, traumatic brain injury (TBI), impaired emotion-specific processing, and personality or communication disorders (33–41). The (partial) independence of HRV parameters from conscious experience also makes application possible when the requirements for active collaboration need to be limited (e.g. during monitoring) or continuous collaboration is questionable even in simple experimental paradigms (e.g. in subjects with severe brain damage). In this respect, the approach appears to be suitable for privileged application in the study of subjects with severe disorder of consciousness, such as those in a VS or MCS.
HEART RATE VARIABILITY AND BRAIN INJURY
Two patterns of autonomic hyperactivity have been described, namely a paroxysmal sympathetic hyperactivity in the absence of parasympathetic major contribution, and the combined sympathetic/parasympathetic hyperactivity (“mixed autonomic hyperactivity disorders”) (42). Non-neurological organ dysfunction (with paroxysmal sympathetic hyperactivity resulting in respiratory/cardiovascular dysfunction) seems to be associated with brain injury (43, 44) and the risk of death increases in patients with severe cardiac uncoupling and depressed HRV (45, 46). Sympathetic hyperactivity and over-responsiveness to afferent stimuli have been observed in a HRV study on TBI patients with dysautonomia (42, 47–50). A parallel increase in the vagal activity and intracranial pressure (possibly due to compression of the vagal nuclei or brainstem) has been documented in patients changes in the LF power (51–53). A significant decrease in the LF/HF ratio was observed in TBI children at intracranial pressure above 30 mmHg (54). Lowensohn et al. (55) observed a HRV decrease with rising intracranial pressures in subjects with severe brain injury. Subacute studies have shown comparable changes in the LF/HF ratio compared with controls or a decrease in the HF power (56, 57) (Table II).
|
Table II. Heart rate variability (HRV) and brain injury
|
|
Author
|
Subjects
|
Results
|
|
Perkes et al., 2010 (42)
|
349
|
The core clinical features of PSH-heart rate were correlated with, blood pressure, respiratory rate, temperature, sweating, and motor hyperactivity.
|
|
Riordan et al., 2007 (46)
|
4,116
|
Reduced HRV was associated with an increase in mortality; beta B exposure appears associated with increased survival across all stratifications of cardiac uncoupling.
|
|
Riordan et al., 2009 (45)
|
2,178
|
Reduced HR multiscale entropy was significantly associated with increasing mortality and is a reliable predictor of mortality in TBI patients.
|
|
Baguley et al., 2009 (50)
|
27
|
HRV measures differentiate between (TBI) subjects with normal and elevated autonomic activity. HRV and event-related heart rate changes help in the diagnosis of dysautonomia. The comparison of HRV and heart rate parameters suggested an over-responsivity to nociceptive stimuli in dysautonomic subjects.
|
|
Kawahara et al., 2003 (51)
|
42
|
HRV analysis showed enhanced parasympathetic activity, probably associated with increased intracranial pressure in patients with acute subarachnoid haemorrhage.
|
|
Mowery et al., 2008 (51)
|
291
|
Cardiac uncoupling increases with ICP, cardiac uncoupling and ICH predict mortality.
|
|
Morris et al., 2006 (53)
|
1,425
|
Reduced heart rate variability is a new biomarker reflecting the loss of command and control of the heart (cardiac uncoupling).
|
|
Keren et al., 2005 (56)
|
20
|
Change towards HRV normalization predicts recovery of the autonomic nervous system in patients with TBI.
|
|
HR: heart rate; PSH: paroxysmal sympathetic hyperactivity; TBI: traumatic brain injury; ICP: intracranial pressure; ICH intracranial hypertension.
|
HEART RATE VARIABILITY AND PREDICTION OF OUTCOME
HRV has been proposed as a useful predictor of outcome in brain-injured patients (27, 58, 59). Reduced LF/HF ratios have been associated with low scores on the Glasgow Coma Scale and increased risk of brain death (54). A correlation between LF, severity of neurological dysfunction and outcome has been reported in TBI children (60, 61) and adults (62). The global HRV and parasympathetic tone were higher in TBI patients who later died than in those who survived; during the awaking period, the global HRV and parasympathetic tone were lower in those patients whose neurological condition later worsened compared with patients with a good recovery (58, 62–64). Attenuated parasympathetic tonus and low HF were found to correlate with the severity of brainstem damage, while very low LF and HF power was associated with progression towards brain death in TBI patients (65). Amelioration of the HRV total power in the first 3 months after TBI was correlated with recovery of autonomic function in a prospective study (56). Changes in autonomic reactivity, namely decrease in parasympathetic activity (normalized unit of high-frequency (nuHF)) and increase in sympathetic activity (normalized unit of low-frequency (nuLF)), were found to parallel the recovery of consciousness in TBI patients (66) (Table III).
|
Table III. Heart rate variability (HRV) and prediction of outcome
|
|
Author
|
Subjects
|
Results
|
|
King et al., 2009 (58)
|
75
|
HRV triages and discriminates the severely brain injured patients during helicopter transport better than routine trauma criteria or en-route pre-hospital vital signs.
|
|
Cooke et al., 2006 (59)
|
84
|
Heart period variability analyses discriminate patients with poor prognosis (death) from those surviving TBI.
|
|
Biswas et al., 2000 (54)
|
15
|
HRV power spectral analysis (e.g. LF/HF ratio) as a useful ancillary test in determining the severity of brain insult and prognosis in children with traumatic brain injury.
|
|
Goldstein et al., 1993 (60)
|
11
|
Damaged sympathetic cardiovascular system in children with severe brain injury and complete interruption of the autonomic cardiovascular pathways in brain death.
|
|
Goldstein et al., 1996 (61)
|
36
|
Sequential changes in heart rate, respiratory rate, blood pressure, heart rate power spectra, and plasma catecholamine concentrations in patients with acute brain injury identify disruption of the autonomic nervous system control on heart rate proportionally to the degree of neurological insult in children with brain injury.
|
|
Rapenne et al., 2001 (62)
|
20
|
HRV provides useful information in the early prognosis of patients with severe brain trauma.
|
|
Norris et al., 2005 (64)
|
1,316
|
HRV independently predicts death in TBI patients and detects early differences in the mortality rate of groups of patients.
|
|
LF: low frequency; HF: high frequency; TBI: traumatic brain injury.
|
HEART RATE VARIABILITY AND RESPONSIVENESS
HRV measures are used to assess the contributions of the autonomic nervous system in sustaining consciousness and its functional re-organization during recovery in subjects with severe disorder of consciousness. The nuLF descriptor of sympathetic activity was found to increase in VS subjects interacting with relatives (the “mom effect”) (Fig. 1) in the absence of any activation in control conditions (67). Higher HRV and HF values were recorded in a comparable study (68, 69), with minor differences conceivably depending on different stimulus paradigms and HRV data processing (70). Consistent patterns of variation in HRV (e.g. in the nuLF values) were observed in healthy controls and TBI patients listening to classical music of different authorship aimed at evoking distinct emotional responses. The responses were classified as “positive” or “negative” based on the controls’ subjective reports; the nuLF patterns during listening differed from baseline and among musical samples, with a relationship with the music structure (71). Changes in the HRV patterns comparable to those observed in brain-injured subjects and in controls were detected in the same experimental conditions in subjects unambiguously diagnosed as being in a VS (72–74) and a relationship was observed between the HRV nuLF and LF peak and the occurrence of a visual pursuit response, a neurological marker of the subject’s evolution from the VS to the MCS (75–77) (Table IV).
Fig. 1. The “mom’s effect” in a subject in the vegetative state (VS): heart rate variability measures (fast Fourier transform (FFT) and auto regressive (AR)) in resting condition (baseline), while the subject’s mother was trying a personal interaction (test condition) and with an unfamilial person repeating the mother’s approach (control) (71).
Table IV. Heart rate variability (HRV) and responsiveness |
Author |
Subjects |
Results |
Wijnen et al., 2006 (66) |
16 TBI subjects |
Autonomic reactivity provides useful information on the severely damaged brain responsiveness to environmental changes. |
Dolce et al., 2008 (67) |
12 VS subjects |
HRV changes in response to a relative’s presence or voice (the “mom effect”) suggest residual rudimentary personal interaction in VS subjects. |
Gutiérrez et al., 2010 (68) |
Case report |
Auditory stimulation induced recordable changes in HRV in VS subjects, suggesting residual preserved cognitive function detectable by cardiovascular descriptors. |
Machado et al., 2011 (69) |
Case report |
Changes of HRV related to the emotional response to the mom’s voice (the “mom effect”). |
Riganello et al., 2011 (70) |
12 VS subjects |
Modifications in the HRV (nuLF) in response to emotional stimuli (voice of relatives), but not to controls. |
Riganello et al., 2008 (71) |
16 TBI subjects
26 healthy controls |
HRV described autonomic concomitants of emotional responses to complex sensory stimuli with emotional relevance (symphonic music). |
Riganello et al., 2010 (72) |
9 VS subjects
16 healthy controls |
Comparable autonomic changes with emotional relevance were induced by complex stimuli (music) in VS subjects and controls. |
Candelieri et al., 2011 (77) |
7 VS subjects
8 MCS subjects |
Two parameters obtained by HRV analysis (nuLF and peak of LF) proved highly correlated to eye-tracking. |
TBI: tramatic brain injury; VS: vegetative state; nuLF: normalized unit of low-frequency. |
HEART RATE VARIABILITY AND THE CENTRAL AUTONOMIC NETWORK
The central control of autonomic function and the complex interplay between the CNS and the autonomic system and between the sympathetic and parasympathetic subsystems is modulated by direct/indirect descending, ascending and bidirectional connections among neural structures (24, 78, 79). A functional integrated model (usually referred to as the central autonomic network, or CAN) has been proposed and would include cortical components (medial prefrontal, anterior cingulate, and insular cortex), the paraventricular, amygdala central and lateral hypothalamic nuclei, and structures in the midbrain (the periacqueductal gray region) and pons (nucleus of the tractus solitarius, nucleus ambiguus and ventrolateral medulla), with primary outputs from stellate ganglia and vagus nerve to the sinoatrial node of the heart (24, 31) (Fig. 2). Telencephalic structures are connected with the hypothalamus and brainstem and contribute in the control of the autonomic organization (24, 80). The insula (visceromotor area) is involved in the control of sympathetic and parasympathetic outputs (via a relay in the lateral hypothalamic area and through the amygdala) and in the autonomic and endocrine responses and motor activation needed to express the emotional response (78). The anterior cingulated cortex and its projections to the prefrontal cortex, amygdala, hypothalamus and brainstem are involved in the modulation of autonomic output in response to pain and emotional or behaviourally significant stimuli (81). The hypothalamus is thought to integrate autonomic and endocrine responses and to sustain vital homeostatic mechanisms, such as thermoregulation, osmoregulation, response to stress, etc. (82).
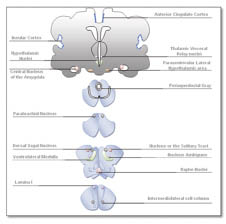
Fig. 2. Schematic outline of the central autonomic network (adapted from Benarroch (92)).
The CAN is essentially a dynamic system, with its activity depending on initial state (83). A functional relationship between HRV measures, the CAN operational status and the activity in the neural structures involved in affective and autonomic regulation has been first suggested by Thayler (84–86). Parasympathetic activation decreases the firing rate of pacemaker cells and HR, while sympathetic activity results in an increase of HR and firing rate of the pacemaker cells in the heart sinoatrial node (87). Autonomic, attentional, and affective systems can be integrated in a functional model with the cardiac vagal tone (23, 88, 89). The autonomic nervous system, in general, and the CAN, in particular, are thought to be indexed by HRV measures.
CONCLUSION
HRV is an output measure with potentially wide application, but its use in neuroscience and medicine is occasionally questioned (90–92). A number of autonomic functional tests, including plasma and urinary catecholamines, provide indirect information on the sympathetic or parasympathetic function (93), and direct measures of sympathetic activity have been obtained from the cardiac norepinephrine spillover and by microneurographic techniques or direct recording from skeletal muscle (94–95). However, these approaches are invasive and inapplicable on large subjects’ samples, and only indirect methods are available today to obtain information on the parasympathetic system (96, 97). In this respect, HRV methodologies benefit from being non-invasive, with high benefit/cost ratio. HRV measures are obtained at limited costs, labour and accuracy of recording and information on the autonomic system functional condition or response, albeit indirect, is obtainable also when voluntary reports would be distracting, in the absence of the subject’s collaboration (as in cases of the severe disorder of consciousness), whenever sophisticated experimental designs and data recording procedures are impracticable (e.g. in the intensive care unit), or when observation needs to be non-invasive and must cause no discomfort (e.g. in psychiatry or in sports medicine), or long-term observation is necessary.
HRV remains a suitable, although indirect, tool to assess residual or emerging sensory/cognitive function and to predict outcome of subjects with severe brain injury, including subjects in a VS or MCS. The CAN model provides an independent approach in the understanding of the HRV measures as descriptors of the integrated function of, and interaction between, the CNS and autonomic (parasympathetic and sympathetic) system. There is evidence of applicability in the study of severe disorder of consciousness.
REFERENCES





