Giuliano Dolce, MD, Lucia F. Lucca, MD, Maria Quintieri, Elio Leto, Stefania Rogano, MD, Francesco Riganello, PhD and Loris Pignolo, Eng
From the S. Anna Institute and Research in Advanced Neurorehabilitation (RAN), Crotone, Italy
Giuliano Dolce, MD, Lucia F. Lucca, MD, Maria Quintieri, Elio Leto, Stefania Rogano, MD, Francesco Riganello, PhD and Loris Pignolo, Eng
From the S. Anna Institute and Research in Advanced Neurorehabilitation (RAN), Crotone, Italy
The operational model and strategies designed for use in the S. Anna – Research in Advanced Neurorehabilitation Institute for the care and neurorehabilitation of subjects in the vegetative or minimally conscious states are described here. A total of 722 patients were admitted, cared for and discharged from the institute in the period 1998–2009. Application of the model approach has progressively shortened the time of hospitalization and rehabilitation and reduced costs.
Key words: severe disorder of consciousness; vegetative state; minimally conscious state; healthcare; neurorehabilitation, outcome.
J Rehabil Med 2012; 44: 512–516
Correspondence address: Giuliano Dolce, S. Anna Institute and RAN – Research in Advanced Neurorehabilitation, Crotone, Italy. E-mail: giulianodolce@libero.it
Submitted September 27, 2011; accepted January 30, 2012
INTRODUCTION
The S. Anna Institute – Research in Advanced Neurorehabilitation (RAN) for the care and neurorehabilitation of subjects with acquired severe brain damage and disorder of consciousness has been operative in Crotone, Italy, since 1998. The institute aims to meet the needs of a local population of 3–4 million; to date it has admitted, treated and discharged a total of 722 subjects. In the process, dedicated units have been designed and set up to care for subjects with different clinical conditions and at different stages of evolution after brain injury. The functional organization and care and neurorehabilitation procedures in each unit have been designed to respond to the subjects’ needs, particularly for those patients who cannot be discharged or treated at home, who need long-term hospitalization. The aim of continuous reorganization since 1998 was to achieve a progressive, cost-efficient reduction in the length of hospitalization in the semi-intensive units for acute patients and in the duration of the rehabilitation protocols, and to help improve outcomes. The objective of this paper is to describe the model and the strategies designed to operate it.
PATIENTS AND DIAGNOSIS
Subjects with severe acquired brain damage and disorder of consciousness are routinely admitted to the institute upon referral from intensive care or neurology/neurosurgery units. There are no pre-determined admission criteria, other than autonomous breathing, stability of vital parameters, and absence of indications for further (neuro)surgery. Patients are classified as being in a vegetative state (VS; also referred to as unresponsive wakefulness syndrome (UWS)) by the current clinical criteria and applicable scales; evolution into a minimally conscious state (MCS)1 (1–6) is diagnosed when reproducible or sustained behavioural patterns associated with evidence of awareness of self or environment are observed (7–11). Outcome is conventionally assessed with the Glasgow Outcome Scale (GOS) (12, 13) despite occasional ambiguities in this scale in the classification of VS or MCS (14, 15).
1The MCS (8–10) was not defined until 2002 and the revised Coma Recovery Scale (7) was not in use in Italy before 2008 (18). Subjects admitted to the S. Anna–RAN in 1998–2002 were initially diagnosed as being in a VS with (“atypical” VS) or without any consistent behavioural responsiveness; in this regard, the Aspen Neuro-behavioral Conference Workgroup guidelines (9, 10) were informally followed. The clinical records have been revised for the present study and the diagnosis of VS and MCS reformulated according to these guidelines, but this re-classification did not change the perspective of the study. The VS is currently also referred to as UWS (19); this label is intended to help characterize a condition with somehow unclear boundaries, that shares aetiology and underlying pathophysiology with the MCS, but differs as to prognosis, medical, legal, or popular perception of the bioethical issues (20), allocated resources, healthcare policies, etc.
A total of 722 patients were admitted in the period 1998–2009. Of these, 503 were diagnosed as being in VS/UWS according to the current criteria; demographics, aetiology and outcome are summarized in Table I. At admission approximately 25% of referred subjects (n = 219; 30.3%) featured some consistent, although not constant, behavioural responses compatible with the diagnostic criteria for the atypical VS or MCS. The percentage is consistent with the reported misdiagnosis between the VS and MCS (up to 25–40%) (16, 17); however, the continuous interaction between the S. Anna Institute and the staff of intensive care or neurology/neurosurgery units in the area appears to be incompatible with such a percentage of error. These subjects’ demographics, aetiology and outcome are summarized in Table I and compared with the subjects in VS/UWS at admission in order to infer about evolution and outcome.
|
Table I. Demographics and outcome of 503 subjects diagnosed and 219 not diagnosed as being in a vegetative state (VS) at admission. The length of time in the intensive care units before admission and in the dedicated semi-intensive care units for VS are shown. The Glasgow Outcome Scale (GOS) ranking classes were: 1 = death; 2 = VS exceeding 1 year in duration; 3 = recovery, with severe disabilities; 4 = recovery, with mild disabilities; and 5 = full recovery or recovery with minimal disabilities not interfering with everyday life (12, 13) |
||||||||||
|
Subjects |
n (%) |
Age, years Mean (SD) |
Time in intensive care unit before admission, days Mean (SD) |
Time in the semi-intensive care unit for VS, days Mean (SD) |
GOS rating at discharge, % |
|||||
|
1 |
2 |
3 |
4 |
5 |
||||||
|
Diagnosed (n = 503) |
||||||||||
|
All patients |
503 |
39 (15) |
58 (45) |
154 (117) |
17 |
16 |
23 |
24 |
20 |
|
|
Post-traumatic |
302 (60) |
29 (14) |
50 (47) |
140 (118) |
5 |
16 |
20 |
29 |
29 |
|
|
Vascular |
160 (32) |
56 (15) |
56 (39) |
144 (113) |
34 |
15 |
31 |
18 |
4 |
|
|
Anoxic-hypoxic |
37 (7) |
45 (19) |
63 (53) |
174 (127) |
45 |
21 |
18 |
9 |
6 |
|
|
Others |
4 (1) |
59 (12) |
34 (16) |
63 (33) |
||||||
|
Not diagnosed (n = 219) |
||||||||||
|
All patients |
219 |
44 (19) |
37 (20) |
74 (72) |
5 |
3 |
16 |
30 |
46 |
|
|
Post-traumatic |
120 (55) |
39 (21) |
39 (22) |
72 (61) |
5 |
3 |
14 |
19 |
59 |
|
|
Vascular |
81 (37) |
49 (18) |
36 (19) |
81 (55) |
4 |
4 |
20 |
44 |
28 |
|
|
Anoxic-hypoxic |
5 (2) |
36 (12) |
40 (24) |
46 (42) |
0 |
0 |
50 |
25 |
25 |
|
|
Others |
13 (6) |
56 (16) |
24 (20) |
61 (46) |
25 |
0 |
0 |
50 |
25 |
|
|
SD: standard deviation; GOS: Glasgow Outcome Scale. |
||||||||||
INSTITUTE STRUCTURE AND ORGANIZATION
The institute units were designed and sequentially organized in compliance with the country regulation, and in order to guarantee clinical care and neurorehabilitation programmes that meet patient’s individual pathophysiological conditions, evolution during rehabilitation, and needs.
Operative units
The following units are operative: a 10-bed (2 rooms with 6 and 4 beds, respectively) semi-intensive care unit, also termed the “Awaking Unit” (Semi-Intensive Care Unit for the severe disorder of consciousness, Fig. 1), is dedicated to subjects with severe disorder of consciousness who meet the criteria for diagnosis of VS/UWS in the acute phase at admission. Three dedicated physicians, 5 therapists and nurses rotate to provide a total of 10 h’ assistance per day. Temperature and humidity are kept constant and sterile air is circulated (8 times/h). All beds can be moved to upright positions to promote the patients’ adaptation to a vertical position and to help recover autonomic balance. The staff schedule and rotation guarantee an overall level of 8-h/day/patient medical, nursing and neurorehabilitation assistance. Each patient is monitored by conventional procedures. All subjects undergo a 3 h/day minimum neurorehabilitation, compatible with their clinical condition and stability. The protocols for neurorehabilitation are purported to: (i) favour the recuperation of circadian rhythms by providing changes in illumination; start feeding with regular timing as early as possible; schedule all activities during the 24-h period; (ii) minimize all problems due to bedding; and (iii) transfer the subject from bed to wheelchair; adapt the subject to an upright position; and start the procedures or assisted mobilization as soon as possible. All subjects are treated regularly in a swimming pool at 38ºC to help counterbalance spasticity and provide the muscle relaxation needed for all rehabilitative procedures to be carried out. The neurorehabilitation protocols include assisted passive mobilization, postural positioning, orthoses, relaxation, stimulation of buccal mucosa, single and group mirror excercises, assisted drawing (Fig. 2a), training in swallowing, training in breathing (clapping, assisted coughing), inhibition of pathological postures, hydrotherapy, automatic walking (Fig. 2b), protocols to withdraw the tracheal cannula, etc. Uni- or multi-modal sensory stimulations are presented regularly to help provide communication with the environment.
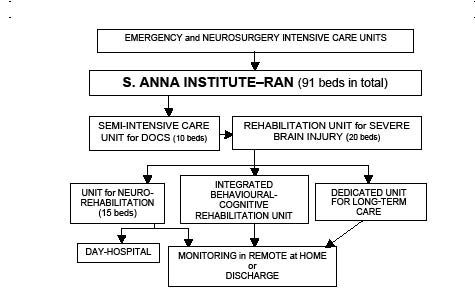
Fig. 1. S. Anna–RAN model for the care and neurorehabilitation of subjects with severe acquired brain damage and disorder of consciousness. RAN: Research in Advanced Neurorehabilitation; DOC: severe disorder of consciousness.

Fig. 2. Examples of rehabilitation treatment of patient with disorder of consciousness in S. Anna Institute–Research in Advanced Neurorehabilitation. (A) Assisted drawing. (B) Automatic walking.
In the framework of the MIMERICA2 project, an ambient intelligence platform combining traditional and innovative sensors for the ambient (temperature and humidity, oxygen, light/dark cycles, noise, etc.) and the relevant functional parameters (body temperature, heart rate and systolic/diastolic blood pressure, breathing, oxygen saturation level, spontaneous movements, voicing, eye movements and blinking, and heart rate variability) of a sub-sample of subjects has been implemented for monitoring. Ambient intelligence collectively indicates pervasive and non-invasive hardware/software infrastructures allowing two-way human interaction with, and full control of, the environment at varying levels of functional complexity. Research into the effects of spontaneous or environment-induced changes in non-neural factors on brain function (e.g. responsiveness) or evolution is in progress. To this end, the platform architecture is interfaced for compatibility and interplay with advanced tools for knowledge management and knowledge discovery, processing data to infer new knowledge and potentiate intelligent processing through intensive and iterative processes (21–23).
2The project and development of MIMERICA were supported by the Italian Ministry of University and Research with dedicated funds for competitive pre-industrial research (2004–2007).
Subjects emerging from the VS/UWS and recovering into a MCS clinical condition (7–9) are transferred to the 20-bed unit dedicated to the patients with acquired severe brain injury (brain injury care in Fig. 1). In this unit, monitoring is limited to the vital parameters, depending on the patient’s clinical needs; and assistance is provided for a total of 7 h/day/patient. Subjects are treated with standard motor, speech therapy and cognitive rehabilitation procedures, depending on the disabilities observed when consciousness is (partially) recovered.
A 36-bed unit is dedicated to the long-term care of patients who have not evolved from a VS/UWS or MCS and are unsuitable for discharge or homecare (long-term care in Fig. 1). Transfer to this unit is made at a time after brain injury that depends on aetiology: 12 months for post-traumatic subjects, 6 for those with major vascular injury and 3 for those who have had massive anoxia-hypoxia. Full nursing and medical assistance, proper feeding/hydration, adaptation to a wheelchair, and passive motor treatments are guaranteed and the possible evolution towards a (partial) recovery of consciousness is monitored by ad hoc protocols. When practicable, the family is trained to be able to take care of the subject at home for limited periods of time, with the aim of re-adjusting the patient to the home environment. Following an increase in the number of beds in this unit from 16 to 36, the turnover along the institute units increased significantly (black vertical bar in Fig. 3) (χ2 = 3.679, p = 0.05).

Fig. 3. Mean length of hospitalization (days) in intensive care or neurology/neurosurgery units of local hospitals and in the Institute S. Anna–Research in Advanced Neurorehabilitation dedicated units. Note how the increased number of patients in institute acute and the long-term units has contributed to reducing hospitalization in local hospitals. The turnover between units increased significantly after increasing the number of available beds in the long-term unit (black vertical bar) (χ2 = 3.679, p = 0.05).
Subjects further evolving from a MCS and (partly) recovering consciousness with residual cognitive impairment and/or behavioural disorders that are incompatible with discharge or untreatable at home are transferred to the 10-bed integrated unit for cognitive-behavioural rehabilitation, with appropriate nursing and psychological support and cognitive rehabilitation.
Subjects (partially) recovering consciousness with residual major motor disabilities are transferred to the 15-bed unit for motor functional rehabilitation and trained to adjust to future, fully-monitored, remote treatment, at home.
This re-organization has progressively reduced the length of hospitalization in the semi-intensive unit for subjects with VS/UWS and has increased the turnover rate, therefore combining an optimal utilization of the institute facilities with the fulfilment of each patient’s needs (Fig. 3).
Work to extend healthcare and neurorehabilitation to patients at home under remote control is in progress. To this end, collaboration between the S. Anna – RAN and the local government and healthcare organization (the Oberon project) has been established, in order to develop and test the potentialities of remote monitoring and homecare of 54 subjects in a persistent VS/UWS or MCS over a 3-year period.
EARLY RECOVERY AND OVERALL OUTCOME
The evolution from VS/UWS to MCS to recovery and the overall outcome were studied retrospectively by referring to two established major descriptors, namely the GOS (12–13) and the re-appearance of a visual pursuit response (24, 25). In general (and in agreement with previous evidence), post-traumatic patients had better outcomes than vascular patients, and anoxic-hypoxic subjects had the worst outcome irrespective of their condition at admission (24–26).
Subjects not in VS/UWS at admission because of the short time between their emerging from coma and their referral to the S. Anna had shorter hospitalization times, both in the intensive and dedicated semi-intensive care units, and better GOS ratings at discharge than those in VS, irrespective of aetiology (χ2= 27.6, p < 0.0001), with a higher probability of scoring a GOS class 5 (χ2 = 11.375, p = 0.0004) and a lower probability of scoring a GOS class 1 (χ2 = 3.309, p = 0.03). Comparable results were obtained when considering post-traumatic and vascular subjects separately (χ2 = 22.26, p = 0.0002, and χ2 = 61.31, p = 0.0001, respectively) (Table I).
Visual pursuit (“the pursuit eye movement or sustained fixation that occurs in direct response to moving or salient stimuli”) is a predictor of favourable outcome, with recovery of consciousness in 73% of subjects in VS/UWS (45% in the absence of eye tracking); it is an established key descriptor of the subject’s evolving from the VS/UWS into the MCS (8–11, 24–27). No differences were observed by testing for a visual pursuit response in the evolution of subjects in VS/UWS due to traumatic or vascular brain injury, who were found to have developed into a MCS in 46% and 49% of cases, respectively, after 50 days. These percentages had increased by 8 months after brain injury, to 89% and 88%, respectively, and had increased further to approximately 90% at discharge or at the end of follow-up (> 235 days). The evolution of subjects with brain anoxia-hypoxia was less favourable, with percentages of evolution increased to a MCS up to 63% at the end of follow-up. Only 12.6% of subjects were diagnosed 8 months after brain injury as still being in a VS/UWS; a later evolution (2 years or more) was observed in 7% of the total group of subjects classified as being in a VS/UWS at admission (25).
The visual pursuit response reflects (partial) recuperation after severe brain injury of the brainstem-cortical interaction and functional organization, which are thought to sustain consciousness and are interfered with by the pathophysiological disconnection resulting in a VS/UWS (25). Its early re-appearance (deemed equivalent to early evolution into a MCS) correlates with a better outcome, confirming the predicting role of this neurological sign (24). However, evolution from the VS to the MCS (at least as indicated by recovered visual tracking) also appears possible several months after brain injury (25).
COMMENT
The extent to which the neurorehabilitative procedures now in use at the S. Anna – RAN Institute or elsewhere are individually or collectively capable of promoting an evolution from the VS to the MCS to recovered consciousness remains, to a relevant extent, undocumented, but a role of the therapeutic milieu, i.e. the synergic effects of the environment and the trainers’ and nurses’ assistance, appears indisputable. Following this rationale, units dedicated to the care and neurorehabilitation of subjects with severe brain injury and consciousness disorders, such as the VS or MCS, are operative in developed countries. The commitment as to resources, logistics, dedicated nursing, rehabilitation and medical care has substantially reduced mortality and the percentage of the so-defined persistent (>1 year) VS. It has improved the chance of favourable outcome, which, in our experience, nevertheless remains worse than for patients with severe acquired brain damage who have never entered into a VS. In our institute, approximately 80% of subjects in VS/UWS due to brain trauma recovered consciousness, while 60% attained recuperation to levels compatible with autonomy or allowing quasi-normal life conditions. To this end, healthcare and neurorehabilitation in dedicated units should be made available as early as possible, with a flexible therapeutic continuum congruent to the functional brain organization attained at each phase during the evolution from coma to a VS or MCS, to recovered consciousness. In our operative model, hospitalization only exceptionally exceeds 6 months, unless cognitive/behavioural disturbances occur after recovery of consciousness. Later evolution from a VS/UWS to a MCS, further improvement to higher levels of functional brain organization, or recovery of consciousness are also possible.
ACKNOWLEDGEMENTS
This study was supported by the Institute S. Anna – RAN. The authors thank Professors Leon Sazbon (University of Tel Aviv, Israel) and Walter G. Sannita (University of Genova, Genova, Italy, and State University of New York, Stony Brook, NY, USA) for continuing support and valuable advice.
The authors have no conflicts of interest to declare.
REFERENCES
