Musculoskeletal ultrasound has gained a significant place in the diagnosis and management of various musculoskeletal disorders due to its several advantages (being convenient, inexpensive, non-invasive, repeatable, providing dynamic imaging and not requiring any exposure to radiation). It has also become a valuable tool in the daily clinical practice of physical and rehabilitation medicine physicians; the musculoskeletal ultrasound probe having become synonymous with the physician’s stethoscope. In this paper, aside from drawing attention to this growing issue in the agenda of PRM physicians, we will touch upon its basic technical features and certain aspects as regards muscle, tendon, ligament, nerve, joint lesions and ultrasound guided interventions.
Key words: ultrasound; physical and rehabilitation medicine; tendon; muscle; nerve.
J Rehabil Med 2012; 00: 00–00
Correspondence address: Levent Özçakar, Hacettepe Üniversitesi Tıp Fakültesi Hastaneleri FTR AD, Zemin Kat, Sıhhıye, Ankara, Turkey. E-mail:lozcakar@yahoo.com
Submitted January 2, 2012; accepted February 15, 2012
INTRODUCTION
The role of musculoskeletal ultrasound (MSUS) in the diagnosis and management of musculoskeletal disorders has increased in recent years, not only in clinical applications but also in research (1, 2). Ultrasonography is convenient, inexpensive, non-invasive, repeatable, and does not require exposure to radiation. Furthermore, because it can provide dynamic imaging and comparison, it now has a paramount role in the diagnostic algorithm of a wide spectrum of diseases in physical and rehabilitation medicine (PRM) (3). However, due to certain barriers (e.g. the lack of an ultrasound device in the majority of PRM clinics and lack of education), only a small minority of PRM physicians is able to use MSUS in clinical practice (1). By highlighting the general principles and applications of MSUS in this paper, we aim to remind PRM physicians of this increasingly useful technique.
DEVELOPMENT AND USE OF ULTRASOUND
History of ultrasound
Ultrasound was utilized first for maritime purposes after the sinking of the Titanic in 1912 to detect icebergs and, later, to identify submarines. The first medical ultrasound was used in 1942 in an attempt to diagnose brain tumours (4). As the diagnostic potential of ultrasound grew, it gained applications in the fields of obstetrics, gynaecology, oncology and cardiology in the late 1950s and early 1960s (5). Although articular and periarticular structures, such as muscles, tendons, cartilage and bone, were first described by ultrasound in 1958 by Dussik, it was not until 1972 when the first diagnostic application of MSUS, to differentiate Baker’s cysts from thrombophlebitis, was published by McDonald (4, 6). Initially, diagnostic ultrasound applications were limited due to poor resolution and lack of real-time imaging capability. During subsequent years, PRM physicians began to lead the medical community with the use of therapeutic ultrasound as a deep-heating modality. In the 1980s, with the use of real-time detailed anatomical imaging, diagnostic musculoskeletal ultrasound became capable of fully evaluating the musculoskeletal system. This is true not only for musculoskeletal physicians, but also for veterinary physicians (7). With revolutionary advances in imaging resolution and capabilities, high-speed digital processing, transducer technology and declining costs, in the past 20 years, the production of high-quality images of musculoskeletal structures has become routine in daily clinical practice, even for the detection of relevant prenatal diagnoses.
Advantages and limitations of musculoskeletal ultrasound
In addition to the aforementioned advantages, MSUS has several other exclusive features in relation to basic radiography (X-rays), computed tomography (CT) and magnetic resonance imaging (MRI), especially in focused musculoskeletal and neurological examinations. Ultrasound is used in a hands-on, interactive examination, which allows the practitioner to use real-time high-resolution soft-tissue imaging. It can be performed conveniently by physicians, and is readily accepted by patients. Rapid side-to-side comparison can be performed easily. This is not only an advantage, but also is an accepted prerequisite for prompt diagnosis among experienced sonographers. A large number of joints in different regions of the body can be imaged rapidly and selectively during a single examination session. MSUS provides the best means of dynamic assessment of the movement of the musculoskeletal system. Changes in various structures can be appreciated readily during active, resisted, and passive motions. In addition, one can perform sonographic palpation or “sonoauscultation” by moving the probe on the most painful area. For example, if the sonographer detects a pathology exactly at the place where the patient indicates (which is also painful during compression with the probe), it is of utmost value for the diagnosis and quite convincing for the patient. Moreover, its high repeatability and sensitivity to change offer potential use in monitoring disease progression and evaluation of the therapeutic efficacy of both local and systemic treatments (8). Above all, it has no known contraindications.
Another important benefit of MSUS in clinical practice lies in guidance for diagnostic and therapeutic interventions. MSUS can be used to assist needle positioning to facilitate invasive procedures, such as aspiration of fluid, drainage of abscess, tissue biopsy and local injection of therapeutic agents. Sonographic guidance is particularly useful when fluid collections are very small or when the trajectory of the needle is adjacent to vital structures (e.g. nerves and arteries) that could be badly damaged during the procedure.
The major limitation of MSUS is that it is user-dependent. Accordingly, experience, and thus education, is mandatory before one can confidently comment on the images of various pathologies. Because sound waves do not penetrate bony structures, a further disadvantage is its limited field of view, potentially resulting in incomplete evaluation of the whole joint anatomy (8). However, this limitation can partly be overcome with certain manoeuvres (e.g. internal rotation of the shoulder for anteriorizing the rotator cuff). Therefore, in short, unless it is inside the bone or covered with bony tissue, almost any structure of the musculoskeletal system can be visualized with ultrasound.
Basic concepts in ultrasonography and equipment
Ultrasonography is based on the emission and reception of sound waves with a frequency greater than the hearing range of the human ear, by piezoelectric crystals located inside the transducer or probe. Depending on the technical features of the particular device, wave frequencies of diagnostic ultrasonography systems generally range from 3 to 25 MHz. Ultrasound waves are transmitted through different structures depending on their composition, and are reflected at the interfaces between materials of different acoustic impedance. Most musculoskeletal work is performed using grey-scale, whereby images are produced in black-and-white format, each white dot representing a reflected sound wave. During the travel of the sound waves; the denser a material is (e.g. bone cortex) the more reflective it becomes and, accordingly, the whiter it appears on the screen. On the other hand, water is the least reflective tissue and it therefore appears as black, while the sound waves travel straight through it. Herein, two factors influence reflectivity: the acoustic impedance of materials and the angle of incidence of the sound beam. Acoustic impedance is the product of a material density and the speed of sound within that substance. According to the intensity of the echo, images are categorized in 3 forms, as follows: anechoic (a structure with high water content that does not produce any internal echoes), hypoechoic (an area that has decreased brightness of its echoes relative to an adjacent structure) and hyperechoic (a structure with low water content, which has increased brightness of its echoes relative to an adjacent structure).
The transducer (probe) is an essential part of the ultrasonography equipment and is responsible for the generation of the ultrasonography beam and detection of returning echoes. A variety of linear-array transducers (large (> 40 mm), medium-sized (< 40 mm) and small-field of view (hockey-stick-shaped)) are currently available in the frequency range used for musculoskeletal examinations. Selection of the most appropriate transducer primarily depends on the frequency (multi-frequency, high-frequency, low-frequency, etc.) whereby high-frequency probes (e.g. 10–25 MHz) are used to visualize superficial structures and low-frequency ones (e.g. 5–10 MHz) are used to visualize deeper tissues.
Improvements in fast digital computer processing and memory storage capacity have recently improved the possibility of applying 3-dimensional (3D) technology to ultrasonography. 3D acquisition can be achieved with ultrasonography using either 2D conventional transducers equipped with a small electromagnetic positional sensor or with dedicated “3D-volume transducers”, which are larger than the standard probes. Although the probes are difficult to handle, they provide better assessment of each scanning plane.
Newer ultrasonography techniques, including colour and power Doppler imaging, provide colour maps of tissues. The amount of colour is related to the degree of blood flow, which may be of use in the assessment of vascular tissues, as in soft-tissue inflammation (8, 9). Power Doppler, which is very sensitive for illustrating inflammation, is commonly used in rheumatic diseases (e.g. for the diagnosis and follow-up of synovitis) (10), traumatic injuries (e.g. healing tendinitis) (11) or in the assessment of mass lesions (i.e. benign vs malignant) (12).
Applications and skills
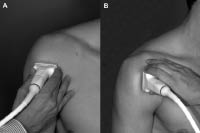
Ultrasonography produces a 2D view of a 3D structure. The ability to skilfully manipulate the probe using specific movements (sliding, tilting, rotating, and heel-toeing) ensures that the targeted structures are examined completely. Because there is gel between the skin and the probe, strict positioning can sometimes be challenging. In this respect, it is better to hold the probe with the first and second fingers (sometimes with the third as well) of the hand being used and let the fourth and fifth touch the patient for stabilization (Fig. 1). The probe should be moved throughout the entire range of the structure to scan substantially and to avoid errors of oversight. Each anatomical area should be explored on various scanning windows (at least two perpendicular planes) so as to obtain all of the necessary information and for appropriate confirmation of a likely diagnosis. If possible, it is suggested that a bony landmark be kept on the screen for better topographic orientation. With experience, a physician can easily develop scanning skills for image optimization and probe manipulation. At this point, a standardized approach to the study of the various anatomical regions is also recommended.
Fig. 1. Positioning the probe for imaging the bicipital tendon, (A) axially and (B) longitudinally, on the anterior side of the right shoulder.
In order to facilitate the detection of abnormalities it is important for physicians to become familiar with the unique appearance of each healthy musculoskeletal structure. An in-depth knowledge of musculoskeletal anatomy is also critical. In this regard, the presence of two textbooks (one on MSUS and the other on anatomy) in the ultrasonography room will always be useful for a sonographer.
Artifacts
An artefact is an image attribute that is not present in the original imaged object. It may be the result of incorrect operation of the imaging equipment, or a consequence of natural processes or properties of the human body. An artefact can be false, multiple or misleading information introduced by the imaging system or by interaction of ultrasonography with the adjacent tissue. While some artifacts reduce the diagnostic power of the scan (anisotropy, reverberation, refraction, speed of sound, beam width, motion, electrical noise and frame buffer dropout), others can be helpful (enhanced through transmission, shadowing, “comet tail”) (13).
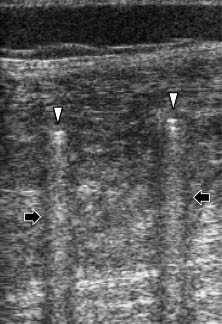
In clinical practice, the artifacts may be used bi-directionally. In the first scenario, the sonographer, while visualizing the condition or structure, recognizes and ignores its relevant artefact (Fig. 2). On the other hand, in the second scenario, the sonographer, without being able to visualize the relevant structure or condition at first glance, initially recognizes the particular artefact and thereafter finds the origin of it (e.g. using the “comet tail” artefact to localize an otherwise unseen small foreign metallic object inside a large compartment).
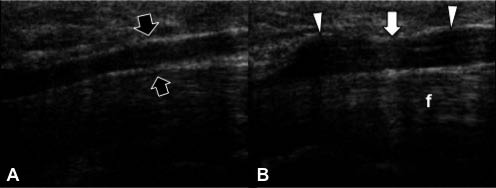
Fig. 2. A 9-year-old boy, who had been operated for Legg-Perthes-Calvé disease, was referred for ultrasonography evaluation of the thigh. The image was obtained by inserting the probe inside the external fixator. The two tips of the screws (white arrowheads) and their hyperechoic “comet tail” artifacts (black arrows) are observed.
The rest of this review will focus on ultrasonography imaging of different components of the musculoskeletal system (muscles, tendons, joints, ligaments, peripheral nerves, cartilage and bones) in various pathologies for which MSUS has improved our diagnostic, follow-up and interventional abilities in daily PRM practice (Table I). The general points that should always be kept in mind by sonographers during MSUS, regardless of the tissue or structure being examined, are listed below:
• the pathology should be viewed in at least two planes;
• the pathology should be confirmed with side-to side comparison (in case of bilateral involvement with that of a normal subject);
• is the structure/tissue present?
• is its shape/size normal?
• is its echogenicity normal?
• is its movement normal?
• is the Doppler evaluation normal?
| Table I. Typical ultrasound appearance of some musculoskeletal structures |
| Structure | Longitudinal view | Transverse view |
| Muscle | “Veins on a leaf” | “Starry night” |
| Tendon | Hyperechoic ribbon-like band | Numerous joined dots in round or oval structures |
| Ligament | Bright, echogenic, linear structure | “Broom-end” |
| Peripheral nerve | Hyperechoic lines with hypoechoic separation in between | “Honeycomb” |
Diagnostic USE OF ULTRASONOGRAPHY
Muscle lesions
Muscles are rather large structures, some even traversing over two joints. Therefore, long linear probes are necessary for their examination. On longitudinal view, the echotexture of a normal skeletal muscle is that of hyperechoic fibroadipose septae (thin, bright, linear bands (“veins on a leaf”) (14) and anechoic contractile tissue. In pennate muscles, these parameters (pennation angle and fascicle length) can even be quantified precisely (15, 16). On transverse view, the septae appear as spot echoes with short, curvilinear, bright lines spreading throughout the hypoechoic background (“starry night”) (14).
MSUS can be used to evaluate a wide spectrum of muscle pathologies, including strain/rupture, haematoma, myositis ossificans (even in the early period when X-rays are non-contributory), myositis, compartment syndrome, rhabdomyolysis, hernia and tumours (17). Its real-time capability is unique in also providing a means to evaluate structures under dynamic conditions. This allows diagnosis of lesions (e.g. hernia) that would otherwise remain obscure. Furthermore, serial follow-up examinations provide valuable information about the healing process and prognosis of the injury. The availability of repeat evaluations can provide the physician with useful information at any time during the course of management.
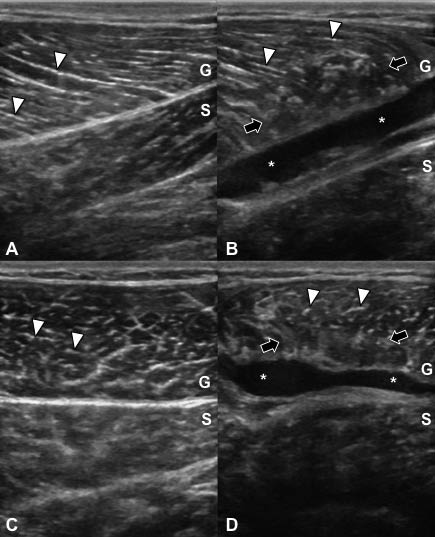
The overwhelming majority of muscle pathologies are traumatic in origin, being either occupational or sports-related. According to the causative factor and localization, the extent of the injury may vary considerably. For instance, in case of a small rupture of the muscle; local haematoma and disruption of the normal muscle architecture may ensue. However, in case of a gross lesion at the myotendinous junction, the resultant haematoma may elongate next to the lining of the outer fascia (Fig. 3). In general, rupture and haematoma are commonly pronounced interchangeably; if there is a haematoma it is likely that there is a rupture and vice versa. In addition, it is worth mentioning that in small lesions, but not in occult ruptures, it is recommended that ultrasonography be performed 24–48 h after the injury and earlier (12 h) for unipennate muscles (18).
Fig. 3. Bilateral ultrasonographic imaging of the gastrocnemius (G) and soleus (S) muscles of a handball player with acute tennis leg. (A, B) Hyperechoic fibroadipose septae (white arrowheads) are observed forming the pennate structure of the gastrocnemius muscle in longitudinal views; (C, D) and in the form of “starry sky” in axial views. (B, D) On the right side of the image, a haematoma (white stars) is observed between the fascia and the abnormal muscle tissue with irregular intrinsic pattern (black arrows).
Tendon lesions
Similar to muscle scanning, the capability of MSUS to provide dynamic real-time imaging makes it the first choice in the diagnosis of tendon pathologies. During the ultrasonographic examination, it is important to apply a correct orthogonal direction to the ultrasonography beam, both for longitudinal and axial views, in order to avoid an anisotropy artefact, which is the dropout of echoes that occurs if the ultrasound beam is not perpendicular to the fibres of a tendon.
Tendons are composed largely of parallel running fascicles of collagen fibres that interweave and interconnect. In longitudinal view, tendons appear as hyperechoic ribbon-like bands, with fibrillar internal structure and marginal hyperechoic line corresponding to the paratenon. In transverse view, they appear as round or oval structures, characterized by numerous closely joined dots, which are homogeneously dispersed, corresponding to the intra-tendinous connective fibres.
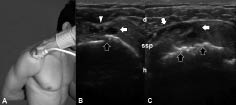
Tendon pathology ranges in severity from tendinosis (tendon degeneration) to intrasubstance or partial-thickness tears and to more severe abnormalities such as full-thickness tears or complete rupture (Fig. 4). Tendinosis occurs as a focal or diffuse process, causing a heterogeneous or ill-defined hypoechoic appearance without loss of tendon volume, whereas partial tears demonstrate a more defined defect within the tendon. A focal, hypoechoic, or anechoic gap or cleft through the tendon is consistent with a full-thickness tear. Non-visualization of the tendon with retraction of its ends is diagnostic of a complete tear. Herein, the sonographer should always be alert for anisotropy in order to avoid misinterpretation as a tendon rupture. In case of tendinitis/tenosynovitis, fluid within the tendon sheath, thickened synovium, loss of the normal fibrillar echotexture, blurred tendon and/or its margins (representing oedema) and increased flow on power Doppler may be present in this condition (19) (Fig. 5).
Fig. 4. (A) Positioning of a patient’s shoulder in internal rotation for better visualization of the rotator cuff in axial view. (B,C) In a 63-year-old lady with shoulder pain, bilateral – full-thickness, partial – supraspinatus tendon (ssp) ruptures are seen as anechoic clefts (white arrows) over the cortical irregularities (black arrows) of the humeral heads (h). (C) Ssp is thicker on the right side and the rupture extends in a wider base on the (subdeltoid) bursal surface. (B) On the left side, the deltoid muscle (d) protrudes towards the rupture while compressing with the probe.
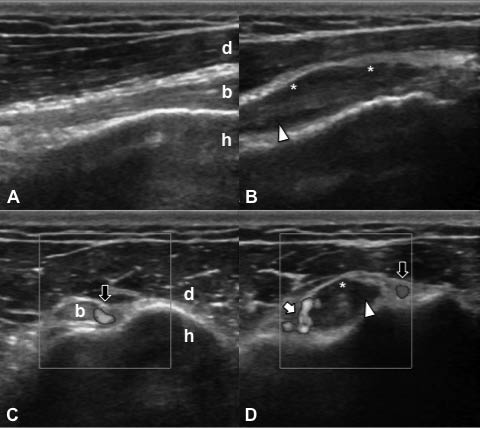
Fig. 5. Bilateral ultrasonographic imaging of the bicipital tendons of a 33-year-old man with bicipital tendinitis. Between the deltoid muscle (d) and humerus (h), the normal bicipital tendon (b) is observed as a hyperechoic band in (A) the longitudinal view and (C) as an ovoid structure in the axial view. (B, D) On the right side of the image, the bicipital tendon is visualized as blurred and oedematous with surrounding fluid (white arrowheads) and synovial hypertrophy (white stars). (D) Doppler imaging reveals increased signal activity, consistent with synovitis (white arrow). (C, D) Black arrows represent normal Doppler signals pertaining to anterior humeral circumflex arteries.
Ligament lesions
Ultrasonography imaging of ligaments is quite similar to that of tendons. A normal ligament is seen as a bright, echogenic, linear structure on MSUS; however, since the fibres of ligaments are more closely aligned, their echotexture is more compact and fibrillar. Ligaments are composed of dense connective tissue, with variability in the amounts of collagen, elastin, and fibrocartilage. Therefore, imaging of ligaments may be more variable than imaging of tendons. Likewise, the typical “broom-end” appearance on axial imaging at the entheseal sites can be used to distinguish ligaments from tendons (20).
Ligaments are best identified by placing the probe between the two bones that the ligaments connect. Ligamentous thickening, heterogeneity, hypoechoic foci, and surrounding oedema or haematomas may be seen in acute ligamentous injuries (sprains) (Fig. 6). Even if the radiograph is negative, small avulsion fractures of the adjacent bone can also be seen on MSUS. Calcifications may be observed in chronic lesions. The dynamic nature of MSUS evaluation can again be useful, in this case, for testing the stability of the ligaments (21).
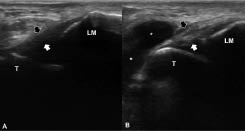
Fig. 6. A 55-year-old veteran athlete evaluated after an ankle sprain. The arrows delineate the anterior talofibular ligament aligned between the lateral malleolus (LM) and talus (T). While it is normal on (A) the left side, it seems to be thickened (but intact) on (B) the right side. In addition, anechoic accumulation of fluid (white stars) is present extending from the sinus tarsi.
In addition, as regards the entheseal sites for both ligament and tendon attachments, ultrasonography imaging plays a crucial role especially in the management of rheumatic diseases, e.g. by visualizing various degrees of tendon, ligament and cortical inflammation or injury (22, 23).
Articular lesions
In a normal joint, bone profiles are seen as hyperechoic lines with shadowing underneath. Hyaline cartilage covering the articular surfaces is depicted as a relatively anechoic, homogenous stripe with smooth contours covering the epiphyses. A minimal amount of fluid can normally be found in the synovial recesses. Synovial tissue lining the joint is immeasurably thin, with smooth, regular contours. Joint capsule is seen as a hyperechoic line. In some cases dynamic assessment of the joint by minimal active and passive movements may help to localize it (24).
In rheumatic diseases, the synovium undergoes significant changes leading to the formation of a mass of synovial tissue; a result of oedema, multiple redundant folds, and villae. The presence of joint, bursal or tendon sheath effusion is used as an excellent, indirect correlate of synovial inflammation. Furthermore, the presence of fluid technically enables better visualization of the synovial thickening, proliferation and villous formation during imaging. In the absence of an effusion, synovitis is diagnosed by the presence of an abnormally thickened hypoechoic region, usually measured in a standard plane with reference to an established normal range or to the contralateral normal joint. Therefore, MSUS can easily detect significant degrees of synovitis that are not determined by clinical examination and can reliably discriminate inflammatory and non-inflammatory joint disease (5, 8, 25). This evaluation has been enhanced on machines with power Doppler setting, which depicts the increased vascularity of the hypertrophied synovium by demonstrating microvascular flow. The Doppler signal can distinguish between active and inactive synovitis, correlating with clinical and laboratory data (26), MRI (27) and histology (28).
Although it is non-specific, joint effusion is a valuable indicator of active joint disease. Ultrasonography has been shown to be one of the best methods for detection of increased intra-articular fluid. Joint effusions are anechoic, compressible, and devoid of Doppler flow (Fig. 7). However, ultrasonography cannot yet accurately differentiate whether a fluid collection is inflammatory, infectious or haematogenous in most cases, and aspiration of fluid, which is more successful with MSUS guidance, remains the gold standard. MSUS can give a basic estimate of fluid viscosity, aiding selection of the appropriate gauge size of the needle for fluid aspiration. Finally, it is important to appreciate that some types of chronic effusions can be mistaken for synovitis, as the fluid will appear hyperechoic and is not easily displaceable by the probe (8).
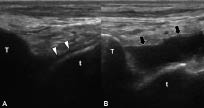
Fig. 7. Comparative ankle joint imaging of a runner: anterior tibio-talar joint, longitudinal view. (A) Articular cartilage (white arrowheads) is observed as a linear anechoic cap overlying the talus (t) on the normal side. (B) On the right side, the accumulation of anechoic joint fluid, indenting the joint capsule (black arrows) is visualized between the tibia (T) and the talus (t).
Nerve lesions
Peripheral nerves are evident on the basis of their position and internal structure. Normal nerves are quite uniform, closely revealing their histological composition on MSUS. On transverse views, nerves are observed as “honeycomb-like” structures composed of hypoechoic dots embedded in a hyperechoic background, and fascicles run longitudinally within/around the nerve. On longitudinal views, nerves typically assume an elongated appearance with multiple hypoechoic parallel linear areas, which correspond to the neuronal fascicles that run longitudinally within the nerve, separated by hyperechoic bands. Nerves are differentiated from tendons by their echotexture, relative lack of anisotropy, location and proximity to the vessels (29).
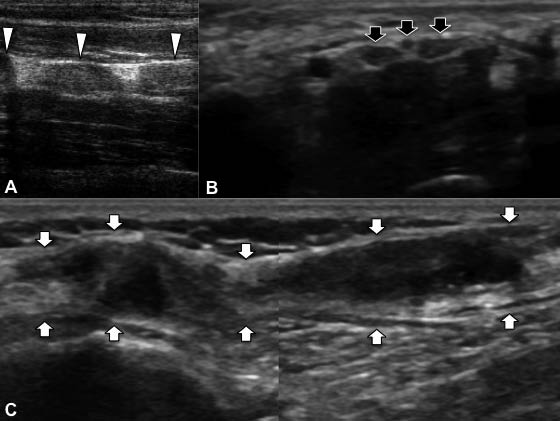
Disorders detected accurately by MSUS include entrapment neuropathies, nerve luxations, masses, neuromas, anatomical variants, inherited and developmental anomalies and traumatic injuries (30–37) (Figs 8 and 9). Noteworthy for the imaging of entrapment neuropathies would be that, with the use of ultrasonography, one can both confirm entrapment (proximal swelling and compression at the site of entrapment), but more importantly, one can also determine the underlying cause (where one exists) (38). Lastly, ultrasonography may be useful during a possible therapeutic injection or, in contrast, sometimes may lead to refraining from intervention but instead referring to surgery.
Fig. 8. A patient who had been operated for carpal tunnel syndrome 15 years previously was evaluated for recurrent complaints. (A) On the palmar longitudinal view, normal median nerve (black arrows) is visualized as an anechoic tubular structure on the left side. (B) The median nerve, over the flexor tendons (f), is observed to be enlarged both proximal and distal (white arrowheads) to the carpal tunnel level (white arrow) on the operated side.
Fig. 9. (A) Longitudinal imaging of an ulnar nerve in a patient with widespread involvement of neurofibromatosis type 1. Anechoic enlargements (white arrowheads) are seen throughout the nerve trunk in the forearm. (B) Median nerve imaging, performed for carpal tunnel syndrome, demonstrates a tri-fascicular nerve structure as a normal variant (black arrows) at the level of the tunnel. (C) Ultrasonographic evaluation of a common peroneal nerve, longitudinal view in a patient with knife injury. The nerve trunk (white arrows) is enlarged inhomogenously, with irregular echogenicities.
DOPPLER ULTRASONOGRAPHY
The capacity of high-frequency colour and power Doppler systems to detect low blood flow and to correlate hyperaemic changes with structural abnormalities has opened new perspectives in the evaluation of a variety of musculoskeletal disorders. There has been significant growth in the application of Doppler systems in the diagnosis and semi-quantification (grading) of synovitis because of its high sensitivity for the identification of increased blood perfusion in the synovium (39). The combination of high-resolution probes and the latest generation of colour/power Doppler workstations allow a clear depiction of even a minimal increase in perfusion in several inflammatory conditions, such as tenosynovitis and enthesitis (40).
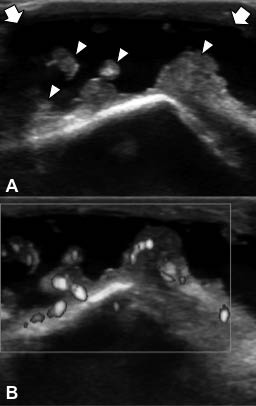
Colour and power Doppler ultrasonography are also of use in characterizing bursitis (Fig. 10), foreign bodies, infections, soft-tissue masses and other soft-tissue inflammatory processes (41). However, optimum technical adjustments as well as probe positioning and compression, which have been discussed elsewhere (42), are necessary for prompt Doppler imaging.
Fig. 10. (A) Grey-scale and (B) ––Doppler imaging of olecranon bursitis in a 67-year-old man with elbow swelling. Anechoic fluid collection (white arrows) and synovial thickenings (white arrowheads) with increased Doppler signal activity are consistent with active synovitis.
ULTRASOUND-GUIDED INTERVENTIONS
The real-time capability of ultrasonography provides a clear advantage in guiding a wide range of musculoskeletal interventions, because the needle can be guided towards its intended target. In other words, if the sonographer is able to see the place/structure, he or she can indisputably reach it. In daily PRM practice, most of these procedures encompass aspiration of fluid collections, injection of various solutions (corticosteroids, local anaesthetics, botulinum toxin (BTX), autologous blood or platelet-rich plasma (PRP), etc.) into joint cavities, tendon/nerve sheaths, para-articular soft tissues or muscles and soft-tissue biopsies (43–45). The selected transducer should be appropriate for the size and depth of lesion. Before starting the procedure, a complete ultrasonography examination (including Doppler examination) of the selected area should be performed not only to detect a precise trajectory, but also to determine the critical structures nearby (such as nerves and vessels) to be avoided in case of potential injuries.
There are two principal techniques: (i) the direct technique, which requires real-time imaging of the needle tip while reaching its target; and (ii) the indirect technique, which refers to a blind injection after detailed measurements have been made as to where and how deep to insert the needle. During the use of the direct technique, according to the local anatomy, the axes of the needle and the probe can be parallel (in-plane) or perpendicular (out-plane). Each technique has its own advantages and disadvantages.
Concerning joint fluid aspirations, it has been shown that ultrasonography guidance is superior to blind interventions, particularly in small joints (46, 47). Especially in chronic effusions, septae may impede full aspiration of the collection and it is not uncommon to finalize an attempt of blind aspiration with a “dry tap”, even if the amount of joint fluid is obvious during inspection. In those cases, ultrasonography guidance (direct or indirect) will definitely provide better navigation for needle insertion(s) inside the compartment(s).
MSUS may be used effectively for several purposes regarding muscle pathologies, some of which include drainage of intramuscular haematomas, intramuscular injection of BTX and PRP. Haematomas are often seen as mixed echogenicity lesions due to the concomitance of liquid, fibrous material and coagulum. They can be drained, preferably with needles 16-gauge or larger due to the thickness of the fluid.
Although BTX injections can readily be performed blindly or with electrical stimulation guidance, MSUS can also be used conveniently for muscles that can otherwise not be promptly delineated. In addition, MSUS has been used to assess the changes in the muscle architecture (thickness, pennation angle, fascicle length) (14).
MSUS plays an invaluable role during the injections pertaining to tendons and ligaments. Depending on the lesion and the material to be injected, the direct method can be used to be sure that the tip of the needle is either inside or outside the tendon. For example, one might wish to place the needle inside a tendon if a PRP solution is to be injected for a resistant tendinosis. On the other hand, one might wish to confirm that the needle is definitely not inside the tendon if a corticosteroid preparation is to be injected for tenosynovitis.
For calcified lesions, ultrasonography can be used for guiding aspirations or during application of shock-wave therapy (40).
FUTURE DEVELOPMENTS
Innovations in ultrasound technology include acoustic microscopy or histosonography, sono-elastography and tissue velocity imaging. Computing advances have allowed very high-frequency probes (> 25 MHz) enabling depiction down to the level of histological details. The possibility of carrying out a histosonographic study in order to understand the histological background of musculoskeletal disorders is attractive (48). Sono-elastography is a non-invasive method in which stiffness or strain images of soft tissues are used to detect or classify mass lesions (49). Tissue velocity imaging refers to detailed quantification of tissue dynamics (strain and strain rate) using sound waves (50).
Three-dimensional and 4D (3D in real-time) MSUS imaging are booming and maturing techniques (4). With continual refinement in image processing, the eventual search for virtual live anatomy may be attained with comprehensive impact. Other recent advances also include new technologies that combine MRI and high-intensity focused ultrasound for confirmative diagnosis (8).
Apart from the above-mentioned technical issues, the role of ultrasonography as an adjunct to electromyography in the diagnosis and follow-up of neuromuscular disorders is worth mentioning. It is more sensitive than electromyography to detect fasciculations and it has recently been shown that even the smaller fibrillations can be visualized with ultrasonography (51). Determining muscle thickness and echo-intensity with computer-assisted grey-scale analysis can be helpful for the detection and differentiation of certain neuromuscular disorders (52).
CONCLUSION
In conclusion, the benefits of MSUS described above provide overwhelming support for its use by PRM physicians in their daily practice. The ultrasound probe can be thought of as synonymous with the physician’s stethoscope. Thus, after taking a substantial medical history and carrying out prompt physical examination, PRM physicians should use the ultrasound probe to make a more thorough examination of their patients.
REFERENCES