OBJECTIVE: There is increasing evidence that robot-assisted treadmill training might be useful for gait rehabilitation after stroke. The aim of this study was to evaluate the muscle activity of stroke patients during robot-assisted walking and overground walking, and of a group of able-bodied subjects during overground walking.
DESIGN: Case-control observational study.
SUBJECTS: Ten stroke subjects and 10 able-bodied control subjects.
METHODS: Electromyography measurements of 7 lower-limb muscles were made in 3 trials: robotic walking, in which stroke subjects walked in a robot-assisted gait orthosis; overground walking for the same group of stroke subjects; and overground walking for control subjects. Trials were compared with respect to electromyography amplitude of selected leg muscles.
RESULTS: Higher muscle activity during overground walking compared with robotic walking was found in several muscles during several phases of the gait cycle. A significant trial × leg interaction revealed smaller differences in muscle activity between the paretic and non-paretic leg during robotic walking compared with overground walking. Furthermore, the muscle activity pattern was not significantly different between control walking and robotic walking, while it was different between control walking and overground walking.
CONCLUSION: Despite lower muscle activity, robot-assisted treadmill training may elicit a more symmetrical pattern of leg muscle activity, which approaches that of able-bodied individuals.
Key words: gait; paresis; electromyography; robotics; walking.
J Rehabil Med 2012; 44: 331–337
Correspondence address: Pieter Coenen, Faculty of Human Movement Sciences, VU University, Amsterdam, Van der Boechorststraat 9, NL-1081 BT Amsterdam, The Netherlands. E-mail: p.coenen@vu.nl
Submitted July 27, 2011; accepted December 13, 2011
Introduction
As a result of stroke, patients often show a decrease in walking speed, stride length and cycle duration (1) as well as an asymmetrical walking pattern (1, 2), which reduces their ability to perform functional activities in daily living (3). Since improvement in walking ability is a major requirement for independence in daily functioning, improvement in gait function is an important aim during the rehabilitation of patients following stroke (4).
Robot-assisted walking devices have been used for a number of years during rehabilitation of stroke survivors for regaining and improving walking ability. The advantages of robot-assisted training, compared with manually assisted treadmill training, are suggested to be a longer training duration, reproducible symmetrical gait kinematic patterns of the leg movements, hands-free operation by a single therapist, and reduction in the physical load imposed upon the therapist (5–8). However, to date, there is no consensus in the evidence on the possible benefits of robot-assisted treadmill training. While some studies report similar or even better training effects when comparing robot-assisted treadmill training with body-weight supported treadmill training (9, 10) or other conventional therapies (11, 12), others report the opposite, with less efficacy for robot-assisted therapy (13, 14) compared with conventional physical therapy. Nevertheless, a systematic review, published in 2007, suggests that stroke patients who receive electromechanical-assisted gait training in combination with physical therapy are more likely to achieve independent walking than patients receiving gait training without these devices (15).
One of the possible disadvantages of robot-assisted walking training may be the guidance of the device, potentially reducing the effort of the patient during training at high passive guidance (16). Another important disadvantage of robot-assisted training is the limited degrees of freedom of a robotic device, which may restrict the walking pattern during robot-assisted walking (e.g. with the device used in the current study, one can only move in the sagittal plane in which pelvis motion is restricted). These restrictions may lead to deviations from a normal walking pattern, which can result in abnormal torque patterns (17), leading to altered muscle activity when using these devices compared with overground walking (18). For example, in able-bodied subjects a higher muscle activity of quadriceps muscles in the swing phase due to the restricted pelvis and a decrease in activity of the ankle flexors and extensors throughout the entire gait cycle as a result of the passive guidance has been reported (18). Because these differences attenuated when subjects were specifically instructed to maximize their effort, it seems that the passive guidance during robot assistance should be kept as low as possible (16). However, the above-mentioned findings are based only on studies on healthy subjects; it seems relevant to investigate whether these results can be generalized to a group of stroke patients, to investigate the possible benefits of using a robotic device for rehabilitation after stroke. Therefore, the present study investigated muscle activity during robot-assisted treadmill walking in stroke patients. This muscle activity was compared with muscle activity during overground walking by the same stroke patients and by a group of able-bodied subjects.
Methods
Subjects
After signing an informed consent, a group of 10 chronic stroke patients (6 men and 4 women, mean age 55 years (standard deviation (SD) 11)) with a left (n = 2) or right (n = 8) hemiparesis participated in the current study. The study was approved by the ethics committee of the VU University, Amsterdam. The hemiparesis was caused by an ischaemic stroke in 5 subjects and by a haemorrhagic stroke in the other 5. The mean time since stroke was 65 (SD 47) weeks. Because the study aimed to compare robot-assisted walking with overground walking, subjects with a functional ambulation category (FAC) score of 5 were selected, indicating that they were able to walk independently on flat surfaces, stairs and slopes without assistance. The patients received conventional physical therapy training prior to the study; however, subjects had no experience of walking in a robotic device. A second group, the control group, comprised 10 able-bodied subjects of similar age (5 men and 5 women, mean age 47 years (SD 12)) without any gait pathologies.
Study design
The group of patients after stroke participated in two measurement trials. During the robot-assisted walking trial (RW), subjects first performed a warm-up and underwent a familiarization protocol consisting of 10 min of robot-assisted walking with maximal body-weight support (BWS) and guidance force (GF). The warm-up phase was followed by a measurement period in which subjects walked in the robot-assisted walking device at a constant walking velocity of 2.2 km/hour. This velocity was the estimated mean overground walking velocity of this subject group, based on the mean walking speed during inpatient therapy. As it has been advised to maximize the subject’s effort during robot-assisted gait training (16), minimal support in terms of GF and BWS was provided; however, both parameters were maintained the same for both legs. These minimal values were determined individually for each subject by gradually reducing both GF and BWS until self-reported maximal effort was reached. During the measurement, 60 s of muscle activity data were collected. After a 10-min break to prevent possible effects of fatigue, the RW trial was followed by an overground walking trial (OW) in which subjects walked without assistance at a self-selected normal walking speed, during which data were collected for 60 s. The control group performed an overground walking trial (CW) at a velocity matched to the RW condition (2.2 km/hour), during which data were collected for 60 s. The CW walking condition was performed using a regular treadmill (Forcelink, Culemborg, The Netherlands) in order to control the walking velocity.
Measurements
For RW, the Lokomat gait orthosis (Hocoma AG, Volketswil, Switzerland) was used. This device consists of a motorized treadmill, a BWS system and two lightweight robotic actuators attached to the subjects’ legs to support the leg movements during gait, allowing GF, BWS and walking speed to be controlled. The exoskeleton leg cuffs of the Lokomat were adjusted to the subjects’ legs to ensure that the subject’s knee and hip joints were aligned with those of the Lokomat. Foot straps were used to prevent unwanted plantar flexion.
A 16-channel electromyography (EMG) recording system with surface electrodes (Porti, Twente Medical Systems International, Oldenzaal, The Netherlands) was used for measuring muscle activity of the following muscles: medial gastrocnemius, tibialis anterior, semitendinosus, rectus femoris, adductor longus, gluteus maximus and gluteus medius. These muscles represent the muscle groups covering the main functions of the lower limbs during gait (e.g. tibialis anterior represents ankle dorsal flexion and the gluteus maximus represents the extension movement of the hip).
EMG signals were sampled at 1 kHz and analysed using custom software (Matlab, Mathworks Inc., Natick, USA). Since it was assumed that there would be differences between the muscle activity of the paretic and non-paretic leg (2), measurements were performed in both legs in the patients group. Only during OW, was video-based gait analysis (SIMI Reality Motion Systems GmbH, Unterschleissheim, Gemany) used to determine the different phases of the walking pattern. In the able-bodied control group, measurements were performed only in the right leg, assuming that the muscle activity in both legs was identical. Heel strikes were determined by a foot-switch (Force Sensitive Resistor, MA-153, Motion Lab Systems, Los Angeles, USA) in all conditions.
Data analysis and statistics
EMG signals were high-pass filtered using a 4th-order Butterworth filter with a 20-Hz cut-off frequency to remove low-frequency artefacts. Data were subsequently rectified and low-pass filtered using a 4th-order Butterworth filter with a 5-Hz cut-off frequency. In all trials, 10 gait cycles were extracted from the collected EMG data and time-normalized to gait cycle duration. Subsequently, for each muscle, EMG patterns were computed, averaging the 10 individual gait cycles to a single gait cycle of the muscle activity for each subject per trial. All above-mentioned calculations were performed using custom-developed Matlab software (version 7.7.0).
Detailed analysis of the muscle activity patterns was performed by dividing the EMG signal into 7 phases of the gait cycle with percentages of duration of the gait cycle (Table I), according to Perry (19). The first phase starts with the initial contact during heel strike, as the end of the gait cycle was the subsequent initial contact of the same foot. Since it is assumed that during CW and RW a normal kinematic walking pattern in the sagittal plane is represented, these percentages were adopted to divide the time-normalized gait cycles into the different phases. A symmetrical gait pattern in able-bodied subjects in terms of kinematics was shown in the 1980s by Hannah et al. (20). A review published in 2000 has shown that, when measuring muscle activity of the lower limbs in healthy subjects, it is reasonable to reduce the amount of data by measuring just one leg (21).
| Table I. Percentages of the 7 phases of the gait cycle |
| Phase in gait cycle | | Percentage of gait cycle |
| Stance phase | | |
| Initial loading | | 0–10 |
| Mid-stance | | 10–30 |
| Terminal-stance | | 30–50 |
| Pre-swing | | 50–60 |
| Swing phase | | |
| Initial-swing | | 60–73 |
| Mid-swing | | 73–87 |
| Terminal-swing | | 87–100 |
However, since patients with hemiplegia after stroke by definition have an asymmetrical kinematic walking pattern, these percentages could not be used during OW. Therefore, the time-normalized EMG signal was divided into 7 phases by means of video gait analysis. The level of asymmetry of the stroke patients was assessed by calculating the stance time ratio (the stance time of the paretic limb divided by that of the normal limb), which is a frequently used measure of gait asymmetry (22). A ratio of one is assumed to reflect perfect symmetry, while a ratio deviating from one reflects gait asymmetry.
Analyses of variance (ANOVA) were performed to investigate possible differences in muscle activity of the paretic and non-paretic muscles between OW, RW and CW during all phases. In case of a significant effect of trial, Bonferroni post-hoc tests were performed. Furthermore, to gain further insight into the overall muscle activity during robot-assisted treadmill walking, the time-normalized EMG signals were divided into two phases: a stance phase from initial loading to pre-swing, and a swing phase from initial swing to terminal swing. Repeated measures ANOVA were performed to assess the influence of the walking trial (RW vs OW) on overall muscle activity in the paretic and non-paretic legs. These analyses were used to test the interaction effect of walking trial and leg (trial × leg) to determine whether the influence of the trial was different between legs in the patient group. All statistical analyses were performed using SPSS (version 17.0.1). For all tests, the level of significance was set at α = 0.05.
Results
During RW, subjects walked with a mean BWS of 45% (SD 22%) of their own weight, while the mean GF on both legs was 45% (SD 16) . During OW, stroke patients walked at a mean speed of 2.8 (SD 0.5) km/h, while during RW the walking speed was set at 2.2 km/h. Furthermore, during OW subjects had a stance time ratio of 0.90 (SD 0.20), tending to deviate from 1.
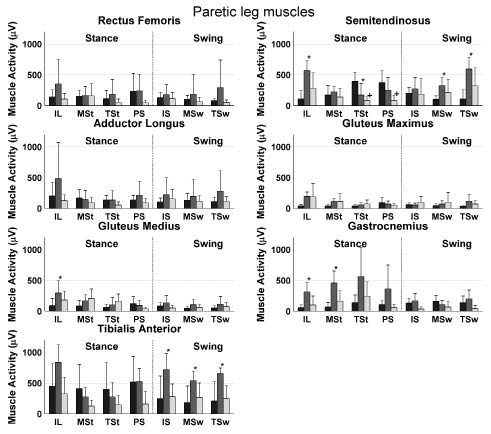
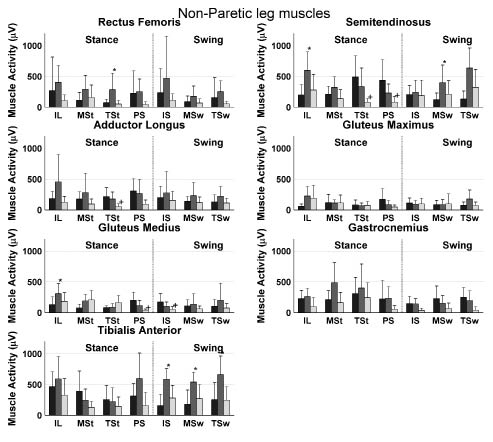
Detailed analysis of muscle activity during the 7 phases of the gait cycle showed higher muscle activities in the non-paretic semitendinosus, gluteus medius, gastrocnemius and tibialis anterior muscle and in the paretic rectus femoris, semitedninosus, gluteus medius and tibialis anterior muscle during phases of OW compared with RW (Figs 1 and 2). Lower muscle activities were found only in the non-paretic semitendinosus in the terminal stance phase of OW compared with RW. Furthermore, differences in phases of RW and CW were found in the non-paretic semitendinosus and the paretic semitendinosus, adductor longus and gluteus medius.
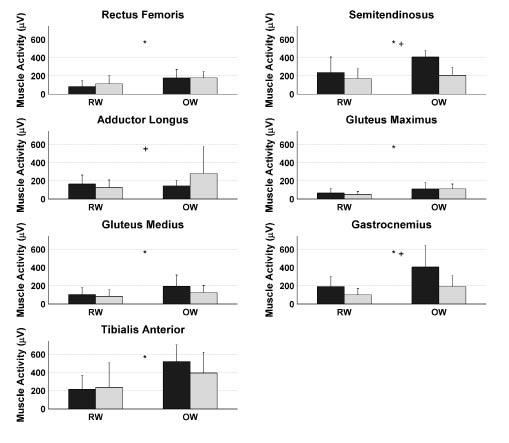
Comparing EMG amplitudes averaged over the entire gait cycle, all muscles, except for the adductor longus muscle, showed a significantly higher activity in OW compared with RW (Fig. 3). Furthermore, significant interactions of the trial × leg were found for the semitendinosus, adductor longus and gastrocnemius muscles.
Fig. 1. Mean muscle activity during robot-assisted walking (dark bars) and overground walking of the paretic muscles (grey bars) and the control group (light bars) during all 7 phases of the gait cycle. Bars represent the mean values averaged over subjects, while the standard deviations are represented by error bars. *Significant difference between the overground walking and robot-assisted walking. +Significant difference between the control group and robot-assisted walking. IL: initial loading; MSt: Mid-Stance; TST: terminal stance; PS: pre-swing; IS: initial swing; MSw: mid-swing; TSw: terminal-swing.
Fig. 2. Mean muscle activity during robot-assisted walking (dark bars) and overground walking of the non-paretic muscles (grey bars) and the control group (light bars) during all 7 phases of the gait cycle. Bars represent the mean values averaged over subjects, while the standard deviations are represented by error bars. *Significant difference between the overground walking and robot-assisted walking. +Significant difference between the control group and robot-assisted walking. IL: initial loading; MSt: Mid-Stance; TST: terminal stance; PS: pre-swing; IS: initial swing; MSw: mid-swing; TSw: terminal-swing.
Fig. 3. Mean muscle activity of the muscles of the paretic (black bars) and non-paretic (grey bars) during the entire gait cycle for robot-assisted walking (left bars) and overground walking (right bars). Bars represent the mean values averaged over subjects, while the standard deviations are represented by error bars. *Significant main effect of trial. +Significant trial × leg interaction. RW: robot-assisted walking trial; OW: overground walking trial.
Discussion
The aim of the present study was to investigate leg muscle activation during robot-assisted treadmill walking in stroke patients compared with that in overground walking by the same stroke patients and by able-bodied subjects. For stroke patients, lower muscle activities were found in different muscles during several phases of RW compared with OW (Figs 1 and 2). Higher muscle activity during RW was found in only one phase of the gait cycle in the semitendinosus muscle. Furthermore, a lower overall muscle activity in all muscles, except the adductor longus muscle was found during RW compared with OW (Fig. 3). This lower muscle activity during RW compared with OW suggests a lower effort during RW than during OW in these muscles, which is probably due to the relatively large support provided by the robotic device. It can be argued that a lower muscle activity reflects better training (e.g. higher efficiency); however, we assume a higher muscle activity to be associated with better training effort leading to facilitation of paretic muscles. The latter because it has been shown that greater intensity of leg rehabilitation improves functional recovery and health-related functional status (23, 24). In addition, a significant interaction effect between trial and leg was found for the semitendinosus, adductor longus and gastrocnemius muscles (Fig. 3). An explanation of this interaction effect is the smaller difference between paretic and non-paretic muscle activity during RW compared with OW, suggesting that the muscle activation pattern is more symmetrical during RW than during OW. This finding might reflect a beneficial aspect of the robot-assisted walking training in stroke patients, since gait symmetry has been used as an important outcome measure in several studies on hemiparetic subjects (e.g. 25, 26) and the achievement of gait symmetry has been assumed to result in functional recovery (2). The stroke subjects who participated in the current study showed a tendency to an asymmetrical walking pattern during overground walking, which supports the importance of these findings.
Comparison with previous findings
The lower muscle activity during robot-assisted treadmill walking compared with overground walking is in line with earlier findings of Israel et al. (16), who showed that a robotic gait orthosis stabilizes the body, reducing muscle activity in quadriceps, hamstrings, tibialis anterior and calf muscles. The pathological gait in stroke patients is characterized by an increased co-activation, especially around the hip, knee and ankle joints (2). Despite the aim of the present study to reduce support during RW to a minimum, GF and BWS were reduced to only half of the full support in some subjects. This support can allow the patient to reduce the muscle activation and maybe the co-activation during RW compared with OW. This relatively high level of support may be caused by the lack of experience of the subjects in robot-assisted walking. Furthermore, it should be noted that, in the present study, similar GFs were chosen for both legs. However, a feature of the robotic device is to apply different GFs on the two legs. The present finding can therefore be applied only to training sessions in which the GF between the two legs is kept constant.
Only during a single phase of the non-paretic adductor longus and the semitendinosus muscle, was muscle activity significantly lower in the CW compared with the RW (Figs 1 and 2). This finding indicates comparable activity patterns of these muscles during RW and OW for stroke patients and able-bodied subjects. However, in a previous study on able-bodied subjects, higher activity of these muscles was found during robot-assisted walking compared with treadmill walking (18). The lower activity of the gastrocnemius and tibialis anterior muscles during RW in the present study (Fig. 3) is in line with the results of this previous study on able-bodied subjects (18). For the gastrocnemius muscle, these differences occur mainly in the first part of stance phase, whereas for the tibialis anterior muscle differences are present during all phases of the swing phase. This effect can be explained by the foot straps fixing the ankle joint during the gait cycle. Foot support during the swing phase will unload the tibialis anterior muscle, while the restriction of plantar flexion in stance will limit the action of the gastrocnemius muscle in push-off. In both paretic and non-paretic gluteus medius muscles, the 3 separate phases of the swing phase tended to be higher, resulting in a significantly higher activity for the entire swing phase during OW compared with RW. Nevertheless, this higher activity can be explained by the restriction of the robot-assisted walking device not to move in the frontal plane.
Furthermore, the present finding indicates that, for stroke subjects, muscle activity patterns are more symmetrical during RW than during OW. This conclusion has also been drawn from a study in a partial body-weight supported treadmill for training stroke subject’s walking ability (27). In the literature, however, consensus on the benefits of symmetry training for patients with a hemiplegic gait pattern has not yet been reached. Although the restoration of gait symmetry does not seem to interact with restoration in functional walking ability (28, 29), gait symmetry is positively related to local stability of walking (30), and gait pattern variability (31). As expected, EMG patterns for stroke patients when walking overground were quite different from those of able-bodied subjects (Figs 1 and 2). Apparently, there are several muscles and phases in which there are differences between RW and OW, but not between RW and CW. Therefore, it can be concluded that the muscle activity in RW approaches that in able-bodied gait.
The present results represent outcomes of a robotic device consisting of robotic-driven exoskeleton actuators, which is just one approach to robotic walking. In the so-called end-effector approach of robotic walking devices (32, 33), the subjects’ legs are not aligned to an exoskeleton, but only the feet are supported by moveable plates that passively move the feet in the swing and stance phase. In this approach legs are not restricted to the exoskeleton gait trajectory, leading to deviating training effects compared with the ones during robot-assisted walking using an exoskeleton approach. In terms of muscle activity these end-effector devices have been shown to lead to muscle activity patterns comparable to those observed during overground walking in healthy subjects (34). In addition, a randomized controlled trial, published in 2007, has shown that robotic-assisted walking using an end-effector approach results in a significant improvement in gait abilities (35). The results of the present study therefore cannot necessarily be generalized to all types of robotic devices.
Methodological considerations
There are some characteristics of the present study that may have influenced the outcome of the study. The self-selected walking speed during OW was 0.6 km/h higher than the walking speed in RW, despite the fact that the RW speed was carefully chosen. However, a study on able-bodied subjects showed no differences in muscle activity patterns during robot-assisted walking with walking speeds ranging from 1.5 to 2.7 km/hour (18). Another study found a proportional increase in muscle activity with an increase in walking speed in able-bodied subjects (36). However, these differences in muscle activity pattern between different walking speeds are mainly affected in amplitude of kinematics and EMG, but not in spatiotemporal characteristics. Based on this, a reduction in the differences in walking speed between the different trials could have decreased the overall difference in muscle activity between the trials. To overcome this source of bias, walking velocity was matched during the RW and CW conditions using a treadmill; the use of a treadmill therefore seems reasonable. Furthermore, the group of stroke patients in this study had a FAC score of 5, which means that they were able to walk for a certain distance without any assistance. Therefore, the present results may not be generalized to subjects with lower FAC scores. Nevertheless, since these more severely disabled patients are more likely to exhibit a more asymmetrical kinematic walking pattern, they might benefit even more from robot-assisted walking training, which may be applicable in a clinical setting.
No randomization of the trials (i.e. RW and OW) was done in the current study; RW was always followed by OW. It can be argued that muscle activity patterns may therefore be biased. For example, patients might have been fatigued during the RW, which may have influenced the muscle activity patterns during the OW. Although it cannot be excluded that the differences in walking speeds between the RW and the OW trials may have led to biases in the present results, the possible effects of fatigue or learning during the trials, which may have biased the results, appear to be small, since the time of exposure to both trials was relatively short and the time of recovery between trials relatively long (10 min).
In conclusion, the results of the present study showed that leg muscle activity of chronic stroke patients is lower in robot-assisted walking than in overground walking. In addition, robot-assisted treadmill training with minimal support/guidance seems to elicit a muscle activation pattern that is more symmetrical and more like that of able-bodied individuals. Despite the relatively low effort during robot-assisted treadmill training, the training seems to have advantages that might make it suitable for rehabilitation of locomotor skills after stroke; for example, the duration of training can be relatively long. These results may be relevant when explaining possible training effects of the current therapy and when providing training strategies. However, no conclusions concerning long-term effects of robot-assisted walking therapy can be drawn from the present study. Whether these results hold for subjects with a more severe hemiplegic gait (i.e. lower FAC scores) and for training situations with less body support and guidance is unknown. Future studies can therefore be directed to answering these questions.
References