OBJECTIVE: To determine whether a single session of anodal transcranial direct current stimulation to the left dorsolateral prefrontal cortex improves attention in patients with traumatic brain injury.
DESIGN: Double-blinded, cross-over design.
Patients: Nine patients with attention deficit after traumatic brain injury.
METHODS: Patients underwent a computerized contrast reaction time task before and after the administration of real transcranial direct current stimulation (2 mA for 20 min) or sham transcranial direct current stimulation (2 mA for 1 min) to the left dorsolateral prefrontal cortex in a double-blind, crossover manner.
RESULTS: Immediately post-stimulation, the transcranial direct current stimulation group showed a tendency of shortened reaction time relative to baseline (87.3 ± 7.8%), whereas the sham stimulation group (122.4 ± 715.5%) did not (p = 0.056). However, this difference was not significant 3 or 24 h after stimulation (p > 0.05). The numbers of correct responses were not changed at any time after stimulation.
CONCLUSION: Anodal transcranial direct current stimulation applied to the left dorsolateral prefrontal cortex improves attention compared with sham stimulation in patients with traumatic brain injury, which suggests a potential role for this intervention in improving attention during cognitive training after traumatic brain injury. A further prospective randomized trial is required to confirm the benefits conferred by transcranial direct current stimulation in this patient population.
Key words: traumatic brain injury; attention; cognition; trans cranial direct current stimulation.
J Rehabil Med 2012; 44: 346–350
Correspondence address: Nam-Jong Paik, Department of Rehabilitation Medicine, Seoul National University College of Medicine, Seoul National University Bundang Hospital, 166 Gumi-ro, Bundang-gu, Seongnam-si, Gyeonggi-do, 463-707, South Korea. E-mail: njpaik@snu.ac.kr
Submitted May 28, 2011; accepted November 22, 2011
INTRODUCTION
In addition to physical disability (1), traumatic brain injury (TBI) leads to cognitive and emotional impairments, such as attention deficit, memory loss, delayed psychomotor and processing speed, and executive and intellectual dysfunction (2), which are devastating to injured individuals and their caregivers (3).
Because attention is a complex mental activity that involves the receipt and initiation of the processing of internal and external stimuli (4), patients with TBI have difficulty focusing on interactions with family members and healthcare staff (3) and coping with the activities of daily living (2).
To improve attention in patients with TBI, pharmacological approaches involving, for example, the administration of psycho-stimulants (5), and cognitive training interventions ranging from simple to more complex tasks (6–9) have been adopted. However, pharmacological stimulants have side-effects, such as nervousness, anxiety and restlessness (5), and the outcome benefits of cognitive training are limited to task-specific improvements (2, 10).
Transcranial direct current stimulation (tDCS), a non-invasive neuro-modulatory modality, is increasingly being used to improve cognitive function in individuals with a range of conditions. For example, excitatory anodal tDCS (11) over the left dorsolateral prefrontal cortex (DLPFC) has been reported to improve attention and working memory (12), planning function, and short-term verbal learning (13, 14) in healthy volunteers, and attention in patients with Parkinson’s disease (15) and major depression (16). Furthermore, it has been reported that one session of anodal tDCS applied to the left DLPFC improves attention in patients with stroke (17).
The frontal lobe is one of the brain areas most vulnerable to TBI due to its anatomical configuration (18) and, therefore, the majority of patients with TBI experience frontal lobe dysfunctions, such as attention deficit and working memory problems (19, 20).
Thus, this study aimed to evaluate whether a single session of excitatory anodal tDCS applied to the left DLPFC also improves attention in patients with TBI. We stimulated the left frontal lobe area because it has been reported that the left DLPF plays a crucial role in attention and working memory (14).
PATIENTS AND METHODS
Patients
Nine patients (8 males, age range 20–78 years) with attention deficit after a traumatic closed brain injury were enrolled. The mean time elapsed between TBI and the study was 216.9 ± 52.5 days (mean ± SE, standard error (SE)) (Table I). No patients had significant brain atrophy on anatomical computed tomography (CT) or magnetic resonance imaging (MRI).
Patients were excluded if they had a metallic foreign body implant, a pacemaker, an artificial cochlear, a history of a seizure event, dementia, cognitive impairment or an unstable medical and/or neurological condition. Patients were also excluded if they were unable to perform the behavioural task used as an outcome measure. Medication taken by patients was maintained throughout the study.
The study protocol was approved by the Institutional Review Board of our institution and written informed consent was obtained from all patients or their legal representatives.
| Table I. Patient characteristics |
| Patient | Sex/age (years) | Brain lesion | Aetiology | Operation | Days after onset | MMSE | First session |
| 1 | M/78 | SDH (bilateral frontal lobes) | Traffic accident | EVD, VP shunt | 246 | 9 | tDCS |
| 2 | M/75 | ICH (left fronto-temporal lobes) | Fall-down | Craniotomy and haematoma removal | 89 | 15 | Sham |
| 3 | M/66 | Haemorrhagic contusion (bilateral frontal lobes) | Fall-down | None | 56 | 13 | tDCS |
| 4 | M/42 | SDH (bilateral frontal lobes) | Fall-down | Craniectomy and haematoma removal | 61 | 13 | tDCS |
| 5 | F/26 | ICH (left fronto-parieto-occipital lobes) | Traffic accident | None | 58 | 14 | Sham |
| 6 | M/27 | ICH, SDH (left fronto-parietal lobes) | Traffic accident | Craniotomy and haematoma removal | 286 | 16 | Sham |
| 7 | M/20 | ICH (bilateral frontal lobes) | Fall-down | Craniectomy and haematoma removal | 258 | 18 | Sham |
| 8 | M/71 | SAH, IVH, ICH (left frontal and cingulate lobes) | Traffic accident | None | 366 | 16 | Sham |
| 9 | M/49 | SDH, ICH (left fronto-parieto-temporo-occipital lobes) | Traffic accident | Craniotomy and haematoma removal | 532 | 13 | tDCS |
| Mean ± SE | 50.4 ± 7.2 | | | | 216.9 ± 52.5 | 14.1 ± 0.8 | |
| tDCS: transcranial direct current stimulation; MMSE: mini-mental status examination; SDH: subdural haemorrhage; EVD: external ventricular drainage; VP shunt: ventriculo-peritoneal shunt; ICH: intracerebral haemorrhage; SAH: subarachnoid haemorrhage; IVH: intraventricular haemorrhage; SE: standard error; M: male; F: female. |
METHODS
Transcranial direct current stimulation
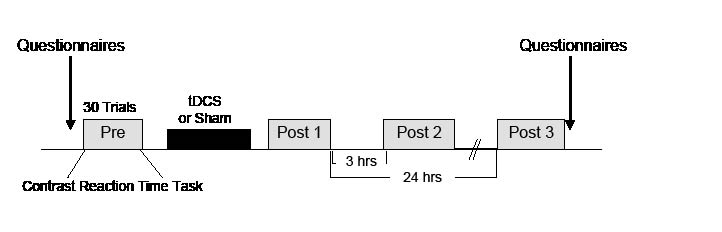
A saline-soaked anode electrode (5 × 5 cm) was placed on the scalp overlying F3, as described by the international 10–20 electroencephalography (EEG) system and corresponding to the left DLPFC (21), and a cathode electrode was located on the contralateral supraorbital area using a battery-driven constant-current stimulator (Phoresor® ΙΙ PM850; IOMED® Inc., Salt Lake City, UT, USA). Each patient underwent anodal tDCS (2 mA for 20 min) or sham stimulation (2 mA for 1 min), separated by at least 48 h in the double-blind, crossover manner described in a previous study (17) (Fig. 1).
Fig. 1. Experimental design. tDCS: transcranial direct current stimulation.
tDCS stimulation was delivered for 20 min, whereas sham stimulation was delivered at the same current level for 1 min and then turned off without the patient’s knowledge. It has been previously reported that sensations of tDCS on the skin dissipate after 30 s of administration (22), and thus, by using this technique we believe that we were able to blind patients to the stimulation type.
Four patients underwent tDCS first and 5 underwent sham stimulation first, in a randomized order. During the experimental procedures, patients and the rater who determined outcome measures were unaware of the type of stimulation administered.
Outcome measures
Before stimulation (Pre) and immediately after the final (Post 3) session, patients were asked to describe levels of attention, fatigue, task difficulty, and sleep quality using a numeric rating scale to demonstrate the possible influence of psychological condition on the outcome measures (range 0–10, where 0 = worst).
A computerized contrast reaction time task (CCRTT) was designed to measure attention using Superlab pro v.4.0 software (Cedrus Corporation, San Pedro, CA, USA). The numbers “1” or “2” were randomly displayed on the centre of a computer screen for 3 s. The patient undergoing testing was then asked to press, as quickly as possible, a “blue” key when “1” was presented, and a “red” key when “2” was presented. Each CCRTT session consisted of consecutive 30 trials, which were performed at baseline (Pre-stimulation), immediately after stimulation (Post 1), and at 3 and 24 h (Post 2 and 3, respectively) post-stimulation. The number of correct responses and reaction times in each session were recorded.
Statistical analysis
The paired t-test was used to compare the baseline values of tDCS and sham sessions. Repeated measures analysis of variance (ANOVARM) was used to test the effects of stimulation type (tDCS vs sham), order of stimulation (tDCS first vs tDCS second), and time (Post 1, Post 2, Post 3). Subsequently, the paired t-test was used to determine whether changes in reaction time and numbers of correct responses had occurred relative to baseline at each time-point (Post 1, Post 2, Post 3). All significance tests were 2-tailed, and p-values < 0.05 were deemed significant. All values were presented as means ± SEs. The analysis was performed using SPSS version 15.0 for Windows.
RESULTS
In terms of perceived levels of attention, fatigue, task difficulty, or sleep quality, no significant differences were observed between tDCS and sham sessions (p = ns; not significant (ns), Table II).
Baseline reaction times (tDCS 1,200.1 ± 223.0 ms vs sham 955.7 ± 114.7 ms, p = ns) and numbers of correct responses (tDCS 23.0 ± 3.0 vs sham 22.2 ± 2.7, p = ns) were also comparable for the two sessions by the paired t-test.
| Table II. Self-perceived attention, fatigue, task difficulty and sleep quality |
| | tDCS | Sham | ANOVARM |
| Pre Mean ± SE | Post Mean ± SE | Pre Mean ± SE | Post Mean ± SE | Intervention effect p-value | Time effect p-value | Intervention × time p-value |
| Attention | 6.7 ± 1.2 | 6.6 ± 1.3 | 7.1 ± 1.0 | 7.0 ± 0.7 | 0.59 | 0.83 | 1.00 |
| Fatigue | 4.6 ± 1.1 | 6.1 ± 1.2 | 6.1 ± 1.2 | 5.1 ± 1.0 | 0.63 | 0.69 | 0.32 |
| Task difficultya | – | 6.1 ± 1.1 | – | 5.6 ± 0.8 | 0.51 | – | – |
| Sleep qualitya | 6.6 ± 1.2 | – | 8.0 ± 0.6 | – | 0.28 | – | – |
| aTask difficulty and sleep quality were analysed using the paired t-test. All criteria were rated using a numeric 0–10 rating (0 = the worst level). tDCS: transcranial direct current stimulation; SE: standard error; ANOVA: analysis of variance. |
Initially, the number of correct responses and the reaction time were not influenced by “intervention”, “time” or “intervention × time” on ANOVARM.
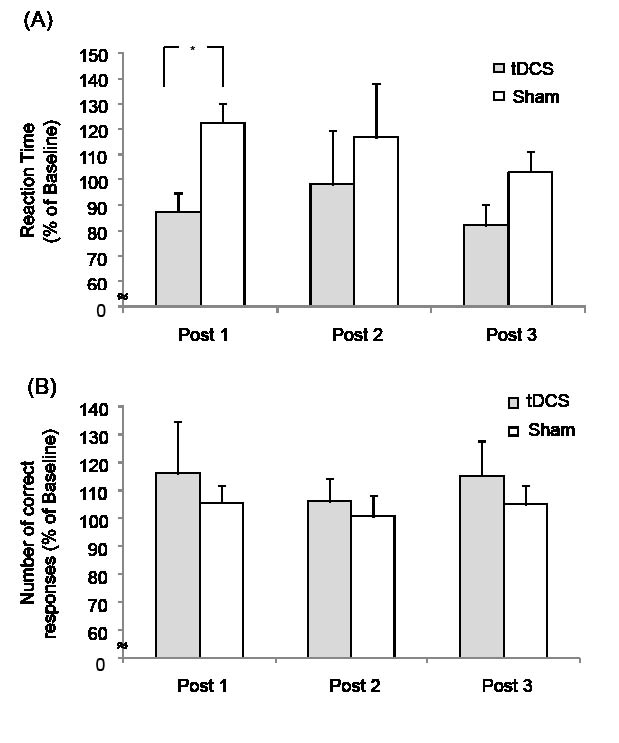
However, mean reaction time at Post 1 was shorter than at baseline after tDCS (from 1,200.1 ± 223.0 ms to 1,001.7 ± 200.8 ms; 87.3 ± 7.8% relative to baseline; p = 0.056 by paired t-test), whereas no change was detected after sham stimulation (from 955.7 ± 114.7 ms to 1,100.4 ± 148.5 ms; 122.4 ± 15.5% relative to baseline p = ns by paired t-test). However, differences between tDCS and sham stimulation were not significant at Post 2 (98.3 ± 21.1% after tDCS vs 116.8 ± 10.9% after sham; p = ns) or at Post 3 (82.1 ± 8.0% after tDCS vs 103.2 ± 14.1% after sham; p = ns) (Fig. 2A).
In terms of numbers of correct responses, no significant differences were observed between tDCS and sham sessions at Post 1 (116.2 ± 18.7% after tDCS vs 105.5 ± 6.3% after sham; p = ns), Post 2 (106.2 ± 8.0% after tDCS vs 100.8 ± 7.4% after sham; p = ns) or Post 3 (115.1 ± 12.7% after tDCS vs 104.8 ± 7.1% after sham; p = ns) by the paired t-test (Fig. 2B).
Fig. 2. Effect of transcranial direct current stimulation (tDCS) on computerized contrast reaction time task results. Effects of anodal tDCS on percentage changes in computerized contrast test results relative to baseline. (A) tDCS stimulation, but not sham stimulation, resulted in a reaction time improvement relative to baseline immediately after stimulation (Post 1, *p = 0.056). (B) No significant improvement was observed in terms of numbers of correct responses after either tDCS or sham stimulation. Bars represent standard errors.
Regarding order of stimulation (tDCS first vs sham first), reaction time and numbers of correct responses were not influenced by “order”, “time”, or “order × time” in ANOVARM, suggesting that the above improvement in reaction time after tDCS was not caused by order of stimulation.
In correlation analysis, improvement in reaction time at Post 3 after tDCS was correlated with mini-mental status examination (MMSE) (γ = 0.67, Pearson’s coefficient < 0.05), but not with age (γ = 0.27, Pearson’s coefficient = 0.48) or gender (γ = 0.32, Spearman’s coefficient = 0.40), which suggests greater improvement in reaction time in the lower MMSE patients after tDCS.
DISCUSSION
Attention deficit is the most common cognitive problem in patients with TBI, and manifests as poor concentration, distractibility, and difficulties with multi-tasking (4). Accordingly, many patients with TBI experience substantial and longstanding impairment in ADL (23). Therefore, cognitive training to improve attention using a systematically and functionally oriented therapeutic rehabilitation approach is important in these patients (24).
Non-invasive neuromodulatory tDCS can modulate cortical excitability and enhance the effects of cognitive training, and thus, potentially, tDCS could be used to supplement cognitive training. Furthermore, repetitive transcranial magnetic stimulation (rTMS), another form of non-invasive cortical stimulation, has been suggested as a potential therapy for TBI (25). However, tDCS has some advantages over rTMS, as it is easier to apply, causes less pain and, importantly, has a lower associated risk of seizure (26). On the other hand, it generally stimulates a more diffuse area of the cortex than rTMS.
Risk of seizure is increased after head injury (27), and this has been a major concern with respect to the adoption of rTMS in these patients. However, tDCS performed within established ranges of intensity and duration is known to be safe (28, 29), and thus, tDCS could be used as an alternative neuromodulatory tool to enhance cognitive function in patients with TBI. In fact, in the present study, no patient reported discomfort or side-effects. However, for safety reasons, we selected only patients with closed TBI, and thus less injury to the blood-brain barrier than patients with a penetrating injury (30).
Anodal tDCS applied to the left DLPFC has been previously reported to improve attention and working memory in healthy subjects and in patients with idiopathic Parkinson’s disease, major depression, or stroke (12, 15–17, 31, 32). Given these results, we considered that tDCS applied to the left DLPFC might also improve attention in patients with TBI. Regarding the mechanism of tDCS, it has been suggested that tDCS depolarizes or hyperpolarizes the membrane potential of the brain tissue and hence induces changes in brain excitability. Rango et al. reported the interesting finding that anodal tDCS over the frontal lobe induced a significant increase in myoinositol content below the stimulating electrode in a proton magnetic resonance spectroscopy study. Therefore it is probable that current changes in the tissue induced by tDCS secondarily causes neurochemical changes in the brain (33).
Attention involves the central executive system (CES) of working memory (34), and can be tested using reaction time tasks, which reflect mental processing time (2, 35, 36). The CES manages multiple complex mental processes under time pressure (37). Generally, patients with TBI tend to show sub-standard performance on timed rather than non-timed tests. Accordingly, in the present study, we used a CCRTT to measure attention. Our results showed significant improvements in speed, but not in accuracy. These findings are encouraging, as they indicate that gains in speed induced by the tDCS were not accomplished at the cost of reduced accuracy. Therefore, such improvements did not rely on a simple movement along an unchanged speed/accuracy trade-off curve.
In the present study, the contrast test was administered repeatedly in a crossover manner, and to exclude the possibility that our findings were affected by the order in which tests were administered, we investigated the effect of stimulation order. The study size was not sufficiently large definitely to exclude the possibility of order effects, but we found no order effect, which suggests that the attention improvement observed after tDCS was not caused by a learning effect. Furthermore, subjective psychophysical levels, for example perceived attention prior to and after the tDCS and sham sessions, were similar.
This study shows for the first time that tDCS could improve attention in patients with TBI. However, the study has limitations. First, in ANOVARM, the number of correct responses and reaction time were not influenced by intervention, time or intervention × time, but anodal tDCS showed an improvement trend in reaction time at Post 1 compared with baseline only through paired t-test, which suggests a modest or weak effect of anodal tDCS over sham in these patients. Secondly, only a single stimulation session was used, and because we found an attention improvement was only achieved immediately post-stimulation, we believe that more stimulation sessions of longer duration or higher intensity may achieve a longer-term effect. Thirdly, we did not use an extra-cephalic reference electrode, and thus, we cannot exclude the possibility that the reference electrode placed over the right prefrontal cortex contributed to our results. Fourthly, the study size was small. Therefore, apparent improvements must be interpreted with caution.
It is probable that surgical procedures could have modified the bone and meningeal anatomy, ultimately altering the current flow in the skull and the amount of charge to the brain. When surgically operated and non-operated patients were compared, no statistical difference in improvement (p > 0.05) was found. We interpreted that the difference in location of stimulation (left DLPF) and operated site might have caused this non-difference in improvement, or that our sample size was too small to demonstrate such difference. However, the possibility of altered current flow in the skull and in the amount of charge to the brain should be considered when applying tDCS in surgically operated patients, hence the different effect of tDCS compared with non-operated patients.
In conclusion, excitatory anodal tDCS applied to the left DLPFC was found to shorten reaction times significantly during a contrast test in patients with TBI, whereas sham stimulation had no discernable effect. This finding suggests that non-invasive cortical stimulation could possibly be used to improve attention during cognitive training after TBI. However, a larger-scale prospective randomized clinical trial is required to confirm this finding.
Acknowledgements
This study was supported by a grant from the Korean Health Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (Grant number: A101901), and by research funds from Handok and Daewoong Pharmaceuticals Co., Ltd and SK Chemicals Ltd.
References