Othmar Schuhfried, MD, PhD, Richard Crevenna, MD, PhD, Veronika Fialka-Moser, MD, PhD and Tatjana Paternostro-Sluga, MD, PhD
From the Department of Physical Medicine and Rehabilitation, University of Vienna, Vienna, Austria
Othmar Schuhfried, MD, PhD, Richard Crevenna, MD, PhD, Veronika Fialka-Moser, MD, PhD and Tatjana Paternostro-Sluga, MD, PhD
From the Department of Physical Medicine and Rehabilitation, University of Vienna, Vienna, Austria
The aim of this educational review is to provide an overview of the clinical application of transcutaneous electrical stimulation of the extremities in patients with upper motor neurone lesions. In general two methods of electrical stimulation can be distinguished: (i) therapeutic electrical stimulation, and (ii) functional electrical stimulation. Therapeutic electrical stimulation improves neuromuscular functional condition by strengthening muscles, increasing motor control, reducing spasticity, decreasing pain and increasing range of motion. Transcutaneous electrical stimulation may be used for neuromuscular electrical stimulation inducing repetitive muscle contraction, electromyography-triggered neuromuscular electrical stimulation, position-triggered electrical stimulation and subsensory or sensory transcutaneous electric stimulation. Functional electrical stimulation provokes muscle contraction and thereby produces a functionally useful movement during stimulation. In patients with spinal cord injuries or stroke, electrical upper limb neuroprostheses are applied to enhance upper limb and hand function, and electrical lower limb neuroprostheses are applied for restoration of standing and walking. For example, a dropped foot stimulator is used to trigger ankle dorsiflexion to restore gait function. A review of the literature and clinical experience of the use of therapeutic electrical stimulation as well as of functional electrical stimulation in combination with botulinum toxin, exercise therapy and/or splinting are presented. Although the evidence is limited we conclude that neuromuscular electrical stimulation in patients with central nervous system lesions can be an effective modality to improve function, and that combination with other treatments has an additive therapeutic effect.
Key words: central nervous disease; electrical stimulation therapy; rehabilitation.
J Rehabil Med 2012; 44: 99–105
Correspondence address: Othmar Schuhfried, Department of Physical Medicine and Rehabilitation, Medical University of Vienna, Waehringer Guertel 18-20, AT-1090 Vienna, Austria. E-mail: othmar.schuhfried@medunivie.ac.at
Submitted November 23, 2010; accepted December 13, 2011
INTRODUCTION
This educational review provides an overview of the clinical application of transcutaneous electrical stimulation of the extremities in patients with upper motor neurone lesions. It is based on a review of the literature and on personal experience. Relevant literature was captured from references in review articles, textbooks and “journal club” meetings. This article focuses on the clinical use of neuromuscular electrical stimulation in conditions such as stroke, traumatic brain injury, multiple sclerosis, cerebral palsy and spinal cord injury. Electrical stimulation is used for the treatment of central nervous system (CNS) lesions with the goal of improving strength and motor control, reducing spasticity and pain, and counteracting the contraction of passive structures (joints, ligaments, tendons). The general purpose of electrical stimulation is to improve the function of the affected extremity.
MODES OF APPLICATION OF ELECTRICAL STIMULATION
A distinction is made between two modes of application of electrical stimulation in patients with CNS lesions: as therapeutic intervention and as a functional substitute.
Therapeutic intervention
Therapeutic electrical stimulation (TES) is used to improve voluntary motor control by strengthening muscles, increasing motor control, reducing spasticity, decreasing pain and increasing range of motion. In TES we distinguish between simple electrical muscle stimulation inducing repetitive muscle contraction and electromyography (EMG)-triggered or position-triggered neuromuscular electrical stimulation in which muscle contraction is triggered by voluntary movement and subsensory or sensory electrical stimulation (submotoric).
Functional substitute
Functional electrical stimulation (FES) provides or assists functional tasks. FES is used directly as a functional substitute. The patient uses the stimulation to execute a function.
MECHANISMS OF ACTION
The postulated mechanism of action for the therapeutic effect of electrical stimulation in CNS lesions is through the facilitation of neuroplasticity of the CNS by increasing the afferent input. Pre-existing functional and non-utilized neuronal connections are activated and/or their inhibition is suspended (1, 2). Electrically mediated repetitive movement facilitates motor relearning to make use of central neuroplasticity. Functional magnetic resonance imaging (fMRI) studies show activation of the somatosensory cortex and supplementary motor area in response to electrical stimulation mediated wrist extension (3–5). Functional improvements are accompanied by increased cortical activation patterns (6). The regular use of a foot drop stimulator strengthens the activation of motor cortical areas and their corticospinal connections, which may explain the improved walking performance even when the stimulator is off (7).
In addition, anti-spastic effects are thought to occur. It is postulated that electrical contraction of paretic musculature leads to reciprocal inhibition of spastic antagonists through the stimulation of spinal interneurones (8).
One form of electrical stimulation is a purely sensory stimulation (submotoric stimulation) that may cause a reduction in muscle tone. The mechanism of action is thought to be an inhibitory effect on spasticity through influencing the excitability of the alpha motoneurones (8) and triggering sensorimotor reorganization (9).
It is discussed that the location of central damage influences the therapeutic effect of electrical stimulation. Sonde et al. (10) reported that, in patients whose basal ganglia were not affected by the lesion, electrical stimulation could have a good effect, whereas no therapeutic effect was achieved in cases of high-grade lesions of the periventricular white matter.
TYPES OF APPLICATION
Electrical stimulation as a therapeutic intervention (TES)
Several modalities of application exist for the use of electrical stimulation as a therapeutic intervention.
“Simple electrical muscle stimulation” consists of direct electrical stimulation of the paretic musculature. The electrodes are placed on the muscle to be stimulated and electrical impulses generate muscle contraction (11). The effect of such stimulation may be enhanced by asking the patient to accompany the motion in terms of thought and, if possible, by actively tensing their muscles. Active tensing of muscles is not indicated when the spastic pattern is aggravated by the patient’s effort towards active contraction. For instance, if when actively attempting to tense the wrist extensors, the fingers are pulled into the spastic flexion position.
Simple electrical stimulation of the deltoid and supraspinatus muscles is performed to reduce subluxation and improve biomechanical integrity and thereby to reduce pain in patients with hemiplegic shoulder pain (12). A randomized controlled trial showed that applying electrical stimulation to the supraspinatus and posterior deltoid muscles in addition to conventional treatment for decreasing the amount of shoulder subluxation in stroke patients is more beneficial than conventional treatment alone (13). However, evaluation of pain showed no significant differences between the groups. A systematic review published in 2008 stated that, so far, there is no evidence to confirm or refute that electrical stimulation around the shoulder after stroke reduces pain. The study designs of the included studies and the electrical stimulation techniques varied and the number of included patients was low. Further studies are required (14).
Reciprocal electrical muscle stimulation of agonists and antagonists in the forearm musculature is also a promising form of electrical stimulation in patients with CNS lesions. The rationale for this is to train reciprocal activation. It may be possible to decrease cortical excitability of the spastic antagonist muscle and to improve the strength of the agonist muscle (15, 16). The indication for this form of stimulation is markedly delayed motor activation of the change in direction of a movement. Specially constructed braces with integrated stimulation electrodes, enabling a cyclic change of stimulation from wrist/finger extensors to wrist/finger flexors are available for this purpose (17). A specially designed hand neuroprothesis system, incorporating 5 electrodes into a brace, provides coordinated hand opening and closing.
Reciprocal electrical muscle stimulation of agonists and antagonists can also be performed with a mesh glove as an anode and two surface electrodes as cathodes. One cathode is placed above the wrist extensors and one on the wrist flexors. By means of manual triggering, the flexors and extensors are activated alternately by electrical stimulation (18). In the lower extremity a reciprocal functional electrical stimulation of dorsiflexors and plantarflexors similar to the timing of the gait can improve the walking ability of chronic stroke patients (19, 20).
During EMG-triggered neuromuscular electrical stimulation of the paretic musculature, electrical stimulation is triggered by voluntary activity of the musculature to be stimulated (6, 21, 22). A prerequisite for this type of stimulation is the ability to tense the paretic muscle voluntarily to the extent that an EMG signal can be measured by a surface electrode (Fig. 1). This corresponds to strength grade 2 on the Medical Research Council (MRC) scale from 0 to 5 (strength grade 2 = motion after elimination of gravity) (23). In most devices, the threshold of the EMG signal to induce an electrical muscle stimulation can be preset and adjusted to strength grade 2. The EMG signal of the muscular activity registered by the surface electrode triggers electrical stimulation. Activation of the appropriate musculature is “rewarded” by an electrical contraction and the movement started voluntarily is concluded by electrical stimulation.
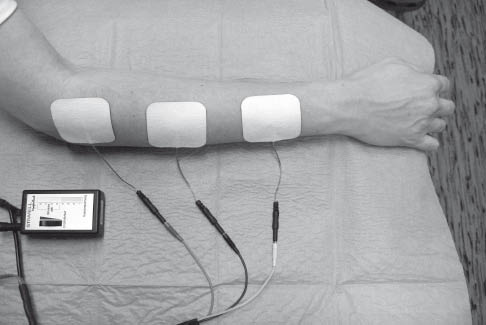
Fig. 1. Electrode set-up of electromyography (EMG)-triggered neuromuscular electrical stimulation. The EMG-signal of the voluntary induced muscle activity is measured by the middle surface electrode.
In contrast to simple electrical muscle stimulation during which the patient follows a pre-given electrical movement pattern, during EMG-triggered neuromuscular electrical stimulation the patient has to start the motion pattern on their own. This therapy requires more personal motor control and cognitive abilities than simple electrical muscle stimulation. According to the clinical symptoms it may be meaningful to start with simple electrical muscle stimulation and, when motor control has improved, switch to EMG-triggered neuromuscular electrical stimulation. This type of stimulation can improve arm function in mild or moderately impaired stroke patients, but not in severely impaired patients (6). A review article found more positive results when electrical stimulation of the upper extremity was triggered by voluntary movement than when non-triggered simple electrical muscle stimulation was used (24).
It has been shown that EMG-triggered neuromuscular electrical stimulation of the lower extremity has a positive effect on motor recovery and walking ability in stroke patients (25, 26).
A further form of feedback-triggered electrical stimulation is position-triggered electrical stimulation (27). The affected extremity is placed in a specially designed dynamic brace with an angle measurement sensor. The patient must achieve a specific angle in a certain direction of motion using the target joint such as the wrist (e.g. 20 degrees of wrist extension from maximal flexion). Once the patient has achieved the angle, electrical stimulation is triggered by the angle sensor (e.g. the wrist and finger extensors). In this form of therapy the patient not only actively tenses a specific group of muscles but also achieves a motion effect of several degrees in the desired direction of motion. One limitation of this form of therapy is that the required technical equipment is difficult to procure, whereas stimulation devices with EMG triggering are easily available for purchase.
Submotoric electrical stimulation does not employ triggering of a motor response. The intensity of stimulation is set in either a sensory or subsensory manner (8). Stimulation is performed on the affected extremity by the use of surface electrodes according to the principle of transcutaneous electrical nerve stimulation (TENS). At the upper extremity the stimulation may be performed in the dorsal and ventral aspect of the forearm. At the lower extremity stimulation is performed on the common peroneal nerve and the sural nerve. Special glove electrodes (mesh glove) or sock electrodes can be used as anodes and the counter-electrodes are used as cathodes on the dorsal and ventral aspect of the forearm or lower leg (Fig. 2) (18).
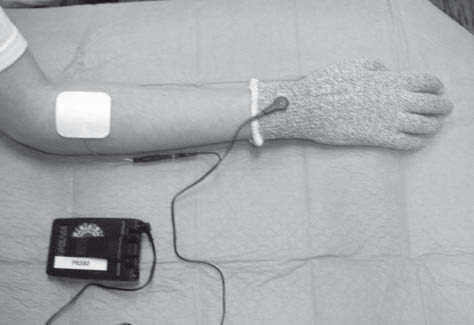
Fig. 2. Electrode set-up of the mesh glove stimulation. The mesh glove electrode is the anode and the counter-electrode in the dorsal aspect of the forearm is the cathode.
The primary purpose of submotoric electrical stimulation is to reduce spasticity and improve motor control through increased afferent inputs (18, 28, 29). TENS can improve the effectiveness of task-related exercise for increasing walking capacity in hemiparetic stroke patients (30). Left-hand somatosensory electrostimulation can enhance the effect of visual scanning training in rehabilitation for post-stroke hemineglect (31).
The therapeutic strategy depends on the existing muscular strength, spasticity, range of motion of the affected limb and the general physical and cognitive condition of the patient.
Pomeroy et al. (32) stated, in their systematic review, that research is needed in order to determine the type of electrostimulation that might be most effective, the optimum dose and the optimum time after stroke.
Electrical stimulation as a functional substitute (FES)
The electrophysiological brace most widely in use is the peroneal nerve stimulator (7, 33–36). By stimulating the common peroneal nerve just below the knee the forefoot is raised by electrical stimulation during the dynamic swing phase of the leg, and the patient’s gait is thus improved (33, 34). Stimulation is activated either by the use of a foot-floor contact transmitter usually placed below the heel (heel switch) (33, 34, 36) or through a motion sensor at the knee (7, 35, 37). One-channel stimulators do not allow differential activation of peroneus and anterior tibial muscles for optimal dorsiflexion-eversion balance. This is possible with two-channel stimulators performing a separate stimulation of the superficial peroneal nerve and the deep peroneal nerve (38). In other applications the first channel is used for common peroneal nerve stimulation and the second channel stimulates other muscles (ankle plantar-flexor muscles, quadriceps muscle, hamstrings or gluteal muscles) at other times in the gait cycle.
In patients with hemiplegia as a result of stroke FES of the ankle dorsiflexors during the swing phase combined with conventional exercise therapy improves walking ability and motor recovery compared with conventional exercise therapy alone (39, 40). To overcome difficulties with transcutaneous electrode placements implantable systems were developed and are undergoing clinical investigations. A randomized controlled trial reported a 23% improvement in walking speed in stroke patients with implanted peroneal nerve stimulators, whereas the improvement in the control group continuing using their conventional ankle-foot orthoses was only 3% (41). In patients with multiple sclerosis significant improvements in walking performance were recorded with drop foot electrical stimulation compared with no electrical stimulation (42, 43). However, compared with a home exercise programme electrical stimulation has a lesser effect on gait aspects (43). Gait improvement in incomplete spinal cord injured patients can be achieved by peroneal nerve stimulation to provoke the flexion response (simultaneous hip and knee flexion and ankle dorsiflexion) (44). The flexion response is also helpful for stroke patients with spasticity leading to a stiff knee during walking. FES-assisted walking accomplished by stimulating the common peroneal nerve and quadriceps stimulation in incomplete spinal cord injured patients helps in restoring walking behaviour and increasing walking speed (45).
A further electrophysiological brace is the leg pacemaker, which is mainly used in spinal cord injured patients. Here the quadriceps muscles and gluteal muscles are stimulated in the supporting leg phase by a manual trigger or a motion sensor so that the patient can stand and walk with the help of electrical stimulation. As an aid the patient uses either forearm supports or a reciprocal walking frame. This procedure requires adequate strength and endurance of the musculature, which must be achieved before the start of FES by strength and endurance stimulation based on a special electrical muscle stimulation treatment (46–50). Standing up and stepping can be improved by optimizing the time delay between the onset of stimulation of the different muscles (48). Before standing up and walking by FES, denervated muscles of paraplegic patients were stimulated daily for a long time (up to 1–2 years), which led to a significant increase in muscle mass and force output during electrical stimulation. This was sufficient to perform FES-assisted standing up (48).
In children with cerebral palsy electrical stimulation has been used to improve gait patterns (51, 52). In combination with task-orientated functional activities neuromuscular electrical stimulation was used to facilitate weak muscles. Significant improvement in the gait efficiency, balance (51) and asymmetrical walking pattern occurred (52).
Upper-limb neuroprosthesis with transcutaneous electrodes in a brace for hand grasp and release have been used successfully in tetraplegia and functional tasks such as pouring from a can and opening a bottle were improved (53). In stroke patients with hemiplegia it is possible to open and close the hand with upper limb neuroprosthesis (54–57). However, in patients with increased hypertonus, in particular, a relevant increase in the function of the upper limb is often not achieved.
THERAPEUTIC STRATEGIES AND PRACTICAL ASPECTS
Intensity and duration of treatment
No uniform guidelines exist with regard to the duration and frequency of single treatment sessions or the overall duration of the treatment (32, 58, 59). In existing studies one may distinguish between two strategies: brief treatment sessions over a period of several months (e.g. 10 min once or twice a day for 3–6 months) (60, 61), or long treatment sessions for a few weeks (e.g. 20–30 min per day for 3 weeks) (62–64). Based on published results, however, it is difficult to decide which time regimen should be given preference. A study by Bocker & Smolenski (60) reports applying electrical stimulation for 2×10 min/day for at least 3 months. In another study it was stated that 6 months of electrical stimulation is necessary for a significant improvement in patients with hemiparesis (61). In acute stroke patients a minimum of 10 h (30 min, 5 times per week for a period of 4 weeks) of neuromuscular electrical stimulation in combination with regular rehabilitation improves the recovery of arm function (62). In acute and subacute (within 3 months post-onset) stroke patients the effect of neuromuscular stimulation of the upper limb for 30 minutes per day, 5 days a week, for 3 weeks persisted at least for 6 months (63). For the lower limb EMG-triggered electrical stimulation of the tibialis anterior muscle for 10 minutes twice a day for 3 months improved the range of motion of dorsiflexion of the foot, the Motricity Index of the paretic leg and functional independence (FIM) in stroke patients. An increase in the stimulation to 6 months did not lead to any further improvement in outcome (60). In a single case study a 20-min EMG-triggered electrical stimulation of the tibialis anterior muscle once every weekday for 4 weeks was enough to produce functional relevant motor, balance and ambulation changes in a chronic stroke patient (64).
With regard to the on/off period it is known that on-times of 2–5 s are sufficient for training movement patterns to enhance strength and an on/off ratio of 1:1 or 1:2 is favourable (65). In order to avoid muscle fatigue when stimulating wrist extensor muscles of patients with hemiparesis the off-period should not be shorter than the on-period (66). The results of this study are more applicable for the stimulation of smaller, more superficial muscles, such as wrist extensors, than for larger and deeper muscle groups of the lower extremity (66).
Application of the electrodes
With regard to the electrode position for electrical muscle stimulation, care should be taken to ensure that the movement axis is correct and uniform activation of the desired target musculature is achieved. In cases of stimulation of wrist and finger extensors, ulnar deviation in the wrist during dorsal extension should be avoided and electrically induced abduction and/or extension of the thumb should be provoked. Depending on the individual situation, electrode dimensions of 5 × 5 cm are usually adequate. In the ankle joint one should ensure adequate counteracting forces on the spastic inversion. It helps to fix the respective electrodes simultaneously over the anterior tibial muscle and the long peroneal muscle. Electrode dimensions of 5 × 9 cm have proved suitable for this purpose (65). To stimulate the peroneal nerve directly, electrodes with a diameter of 2–3.5 cm are placed close to the neck of the fibula at a low threshold point (34, 43).
Table I gives a detailed overview of the parameters of motoric and submotoric stimulation, summarizing the studies of TES and electrode configurations.
| Table I. Stimulation parameters for motoric and submotoric stimulation, electrode configurations | ||
| Parameters | Motoric stimulation | Submotoric stimulation |
| Frequency, Hz | 20–50 | 1.7–100 |
| On-times, s | 2–15 | – |
| On/off ratio | 1:1–1:10 | – |
| Pulse duration, ms | 0.2–0.5 | 0.1–0.3 |
| Waveform | Biphasic preferred | Monophasic or biphasic |
| Configuration of electrodes | For muscle stimulation surface electrodes of 5 × 5 to Surface electrodes with a diameter of 2–3.5 cm to | Surface electrodes of 5 × 5 to 5 × 9 cm are adequate or mesh-glove/stocking electrode |
Therapy goals
Electrical stimulation should be used in patients with CNS lesions without sufficient voluntary motor control of an extremity to perform functional tasks (59). In spastic hemiparesis at the upper extremity the primary goal is to improve function of the hand. The purpose is to enhance strength and volitional activation of wrist and finger extensors by motoric stimulation (with or without EMG-trigger mechanism) and reduce tonus of the wrist and finger flexors. In patients with spinal cord injuries or stroke electrical upper limb neuroprotheses are applied to enhance upper limb and hand function.
In the lower extremity the most common goal is to improve gait function. Drop foot in hemiparetic patients and spastic quadriplegia and paraplegia after spinal cord injury due to various causes are meaningful indications for FES. TES is used when the muscles still possess some residual voluntary activity, but not enough to perform functional tasks.
The primary purpose of submotoric stimulation of the upper as well as the lower extremity is to reduce spasticity and, secondarily, also to improve motor activation (18, 28–30).
Combination with other therapeutic approaches
Electrical muscle stimulation cannot replace exercise therapy and occupational therapy and should always be used in combination with these. This applies to both TES and FES. For example, a dropped foot stimulator triggers ankle dorsiflexion to restore gait function. Gait training sessions are necessary to incorporate this electrically triggered function in an optimal gait pattern. Investigations demonstrate the additive therapeutic effect of combined treatment of this type (30, 31, 39, 40, 60, 62). The effect of combination with botulinum toxin treatment is very meaningful. Antagonist muscle spasticity, e.g. of the finger flexor muscles, often disturbs agonist muscle activity. Focal reduction in tonus of the antagonist muscles by the injection of botulinum toxin may make electrical muscle stimulation possible and effective (67–69). It has been shown that combining splinting with electrical stimulation can reduce spasticity and contractures and improve function (70, 71). There are few publications about the combined use of FES and robotic systems (72, 73). Using a combination of FES and a robotic system driven by the user’s voluntary intention, motion accuracy and arm function were improved in chronic stroke patients (72, 73). Future work will include the application of FES systems to assist the movement of the paretic limb trained with a set of movements in virtual reality (74, 75).
Possible risks, precautions and contraindications
Electrical stimulation may be used in the acute as well as chronic stage of CNS lesions (76). Concerns about spasticity being aggravated by electrical stimulation have not been confirmed in the published literature (76–78). Our own clinical experience to date has also shown that electrical stimulation itself does not increase spasticity. To avoid muscle fatigue in muscle strengthening it is important to optimize the stimulation strategy because a high intensity of stimulation and stimulation frequencies higher than 50 Hz accelerate the rate and level of muscle fatigue (79, 80). As already mentioned, the off-period should not be shorter than the on-period (65, 66). On the other hand, muscle fatigue through electrical stimulation of the spastic muscles might reduce spasticity (81).
For home-based electrical stimulation and long-term use the devices should be easy and safe to operate. An occasional side-effect is skin irritation secondary to long-term stimulation and high current intensities. In patients with epilepsy the indication should be established with great care. In cases of metal implants only biphasic forms of current should be used. Primary contraindications are cardiac pacemakers and implanted defibrillators.
The evidence for the application of TES in the rehabilitation of CNS lesions is still limited. A Cochrane Review has shown that TES improves functional motor abilities compared with placebo (32). The optimal stimulation parameters, dosage, time to start stimulation after the onset of the lesion, and which population will most benefit from each stimulation method remain to be determined. The recommendations made in this article are therefore based partly on personal experience.
In conclusion, a distinction is made between electrical stimulation as a therapeutic intervention (TES) and FES as a functional substitute. Electrical stimulation in patients with CNS lesions can improve the function of the affected extremity. A combination of electrical stimulation with other therapeutic approaches is useful. The mechanism of action is thought to be the stimulation of neuroplasticity through electrical enhancement of the afferent input.
REFERENCES
*This educational review is produced in collaboration with the International Society of Physical and Rehabilitation Medicine.
