Aurore Thibaut, MSc1*, Marie-Aurélie Bruno, PhD1*, Camille Chatelle, MSc1, Olivia Gosseries, MSc1, Audrey Vanhaudenhuyse, PhD1, Athena Demertzi, MSc1, Caroline Schnakers, PhD1, Marie Thonnard, MSc1, Vanessa Charland, MSc1, Claire Bernard, Ir2, Mohammed Bahri, MD, PhD1, Christophe Phillips, Ir, PhD3, Mélanie Boly, Ir, PhD1, Roland Hustinx, MD, PhD2 and Steven Laureys, MD, PhD1
From the 1Coma Science Group, Cyclotron Research Centre and Neurology Department, University and University Hospital of Liège, 2Nuclear Medicine Department, University Hospital of Liège and 3Cyclotron Research Centre, University of Liège, Liège, Belgium. *These authors contributed equally to the writing of this paper.
OBJECTIVE: An extrinsic cerebral network (encompassing lateral frontoparietal cortices) related to external/sensory awareness and an intrinsic midline network related to internal/self-awareness have been identified recently. This study measured brain metabolism in both networks in patients with severe brain damage.
DESIGN: Prospective [18F]-fluorodeoxyglucose-positron emission tomography and Coma Recovery Scale-Revised assessments in a university hospital setting.
SUBJECTS: Healthy volunteers and patients in vegetative state/unresponsive wakefulness syndrome (VS/UWS), minimally conscious state (MCS), emergence from MCS (EMCS), and locked-in syndrome (LIS).
RESULTS: A total of 70 patients were included in the study: 24 VS/UWS, 28 MCS, 10 EMCS, 8 LIS and 39 age-matched controls. VS/UWS showed metabolic dysfunction in extrinsic and intrinsic networks and thalami. MCS showed dysfunction mostly in intrinsic network and thalami. EMCS showed impairment in posterior cingulate/retrosplenial cortices. LIS showed dysfunction only in infratentorial regions. Coma Recovery Scale-Revised total scores correlated with metabolic activity in both extrinsic and part of the intrinsic network and thalami.
CONCLUSION: Progressive recovery of extrinsic and intrinsic awareness network activity was observed in severely brain-damaged patients, ranging from VS/UWS, MCS, EMCS to LIS. The predominance of intrinsic network impairment in MCS could reflect altered internal/self-awareness in these patients, which is difficult to quantify at the bedside.
Key words: vegetative state; minimally conscious state; positron emission tomography; consciousness; self-awareness; traumatic brain injury.
J Rehabil Med 2012; 00: 00–00
Correspondence address: Steven Laureys, Coma Science Group, Cyclotron Research Centre and Neurology Department, University and University Hospital of Liège, 4000 Liège, Belgium. E-mail: steven.laureys@ulg.ac.be
Submitted September 29, 2011; accepted December 5, 2011
INTRODUCTION
The assessment of consciousness in severely brain-damaged patients remains a major challenge (1). For clinicians, consciousness has two main components: arousal (i.e. wakefulness or vigilance) and awareness (i.e. comprising all subjective perceptions, feelings and thoughts) (2). Awareness has recently been subdivided into “external or sensory awareness” (i.e. perceptual awareness of the environment) and “internal or self awareness” (i.e. stimulus-independent thoughts, mental imagery, inner speech, daydreaming or mind wandering) (3). At the bedside, arousal is typically measured by examining eye opening. External awareness is assessed by showing the presence of reproducible command following or “non-reflex”/voluntary movements (4). After severe brain damage and the acute setting of coma, 4 different clinical entities can be disentangled: (i) patients who “awaken” but remain without reproducible signs of command following (i.e. vegetative state (VS), now also called “unresponsive wakefulness syndrome” (UWS) (5); (ii) minimally conscious state (MCS) patients showing reproducible, albeit fluctuating, signs of consciousness, but without functional communication (6); (iii) patients who emerge from MCS (EMCS) recovering functional communication or object use (6); and (iv) locked-in syndrome (LIS) patients who are fully aware yet completely paralysed with the exception of small eye-movements permitting an eye-coded communication (7).
The behavioural assessment of consciousness in non-communicative brain-damaged patients is difficult because movements can be very small, inconsistent and easily exhausted (8, 9). This issue is further complicated when patients have underlying deficits in the domain of verbal or non-verbal communication functions, such as aphasia, agnosia or apraxia (4, 10, 11). Quantifying internal or self-awareness is even more difficult than the assessment of external awareness in these patients. Most, if not all, of the employed consciousness scales mainly assess command-following or the presence of non-reflex movements (i.e. orientation to pain or visual pursuit) (12, 13). Regarding the latter behaviour, some scales, such as the Coma Recovery Scale-Revised (CRS-R) (14) explicitly require the use of a mirror (15), hence possibly assessing some form of self-recognition/internal awareness. Similarly, presentation of the patient’s own name, another auto-referential attention-grabbing stimulus, has been employed by some consciousness scales (e.g. the Wessex Head Injury Matrix (16)). Most behavioural scales, however, mainly, if not totally, assess external or sensory awareness and give little or no information about any possible form of internal or self-consciousness (17).
Recent studies have started to identify the neural correlates of internal and external awareness. An increasing body of evidence, mainly coming from functional neuroimaging (positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) studies) and electrophysiology point to the critical role of a widespread fronto-parietal network in the emergence of conscious awareness, also called “global neuronal workspace” (18–20). Within this widespread fronto-parietal network, two separate systems can be identified: (i) an extrinsic/lateral network encompassing lateral parietal and dorsolateral prefrontal cortices, mainly related to external awareness (i.e. stimulus-dependent or perceptual awareness of the environment) and (ii) an intrinsic/midline network encompassing midline precuneus/posterior cingulate and mesiofrontal/anterior cingulate cortices, mainly related to internal awareness (i.e. stimulus-independent thoughts and self-related thoughts) (3). Given our clinical limitation to objectively measure internal awareness, we here employed objective brain metabolism data obtained from PET in patients with disorders of consciousness (i.e. VS/UWS, MCS, EMCS) and conscious LIS and controls, aiming to measure differences in activity in extrinsic and intrinsic network activity.
METHODS
Brain metabolism was studied by means of [18F]-fluorodeoxyglucose-PET (FDG-PET). The clinical diagnosis was based on the best response obtained by repeated CRS-R (14) assessments the day of the PET study and the two days before and after the PET acquisition. We applied the diagnostic criteria, as published by the Multi Society Task Force on PVS (21), the Aspen Neurobehavioral Conference Workgroup (22) and the American Congress of Rehabilitation Medicine (7). Exclusion criteria for the present study were: (i) the presence of pre-morbid neurological disease; (ii) the presence of ambiguous behavioural signs not permitting reliable clinical diagnosis; (iii) the presence of large structural brain damage exceeding 25% of the whole brain volume not permitting reliable spatial normalization to the standardized stereotaxic brain template; and (iv) the absence of good quality PET data not permitting reliable image reconstruction or correction for attenuation. The control population consisted of age-matched healthy volunteers (n = 39; mean age 45 years (median 45) (range 18–80); 18 men).
FDG-PET data were acquired after intravenous injection of 5–10 mCi of FDG on a Siemens CTI 951 R16/31 scanner (as described in 23) at the University Hospital of Liège, Belgium. Data were pre-processed and analysed using Statistical Parametric Mapping (SPM8; http://www.fil.ion.ucl.ac.uk/spm) as described elsewhere (24–26). In brief, FDG-PET data from each subject were normalized to a standard stereotactic space (using a spatial template adapted to severe brain damage, as previously described in 27) and smoothed with a 14-mm full-width half-maximum isotropic kernel. The design matrix included the VS/UWS, MCS, EMCS and LIS patients’ and control subjects’ scans. Global normalization was performed by applying proportional scaling. The analyses identified brain regions where glucose metabolism was lower in each patient population compared with the control group. The resulting set of voxels values for each contrast, constituting a map of the t statistics (SPMt), was transformed to the unit normal distribution (SPMZ) and thresholded at p < 0.001. Results were considered significant at p < 0.01 family-wise correction for multiple comparisons. Next, we identified brain areas showing a linear correlation with CRS-R total scores. Here, results were thresholded for significance at p < 0.001 with small volume correction (8 mm radius) for multiple comparisons around the previously identified areas (24–26).
Informed consent was obtained from all control subjects and for LIS and EMCS patients, and from the legal representative of all non-communicative patients. The study was approved by the ethics committee of the University and University Hospital of Liège, Belgium.
RESULTS
A total of 132 patients were prospectively enrolled, of whom 62 were excluded because of: (i) pre-morbid neurological disease (8 patients); (ii) ambiguous behavioural signs not permitting reliable clinical diagnosis (12 patients); (iii) large structural brain damage (19 patients) and (iv) technical problems related to the FDG-PET acquisition (23 patients). Hence, 70 patients of the initial cohort were included for further analysis: 24 VS/UWS (mean age 51 years (median 50.5) (range 20–78); 10 men, 2 traumatic), 28 MCS (mean age 41 years (median 36.5) (range 17–81); 19 men, 16 traumatic), 10 EMCS (mean age 41 years (median 41) (range 14–76); 8 men, 4 traumatic) and 8 LIS (mean age 40 years (median 43) (range 22–53); 2 men, 1 traumatic). Patients were studied after a median of 26 months (interquartile range 24 months). Demographic and clinical data are summarized in Table I.
|
Table I. Condt.
|
|
State
|
Age, sex
|
Etiology
|
Time of PET
|
Audition
|
Visual
|
Motor
|
Verbal
|
Comm
|
Arousal
|
|
MCS 5
|
33, M
|
ARCA
|
39.5 months
|
Startle reflex
|
Visual pursuit
|
Automatic motor reaction
|
Vocalization
|
None
|
Without stimulation
|
|
MCS 6
|
64, M
|
Aneurysm
|
6 months
|
Consistent movement
to command
|
Object recognition
|
Automatic motor reaction
|
Intelligible verbalization
|
Intentional
|
With stimulation
|
|
MCS 7
|
50, F
|
Aneurysm
|
28 days
|
Startle reflex
|
Visual pursuit
|
Flexion to pain
|
Oral reflexes
|
None
|
Without stimulation
|
|
MCS 8
|
38, M
|
Anoxia
|
4 months
|
Reproducible movement
to command
|
Visual pursuit
|
Flexion
to pain
|
Oral reflexes
|
None
|
Without stimulation
|
|
MCS 9
|
81, M
|
meningitis encephalopathy
|
46 days
|
Localization ton sound
|
Visual pursuit
|
Localization to pain
|
Oral reflexes
|
None
|
Without stimulation
|
|
MCS 10
|
19, F
|
Traumatism
|
30 months
|
Startle reflex
|
Visual pursuit
|
Flexion
to pain
|
Vocalization
|
None
|
Without stimulation
|
|
MCS 11
|
46, M
|
Traumatism
|
17 months
|
Startle reflex
|
Visual pursuit
|
Flexion
to pain
|
None
|
None
|
Without stimulation
|
|
MCS 12
|
36, M
|
Traumatism
|
270 months
|
Reproducible movement
to command
|
Visual pursuit
|
Automatic motor reaction
|
None
|
None
|
Without stimulation
|
|
MCS 13
|
29, M
|
Traumatism
|
46 days
|
Startle reflex
|
Visual pursuit
|
Flexion
to pain
|
Oral reflexes
|
None
|
Without stimulation
|
|
MCS 14
|
50, F
|
ARCA
|
65 days
|
Reproducible movement
to command
|
Blink
to threat
|
Flexion
to pain
|
Vocalization
|
None
|
With stimulation
|
|
MCS 15
|
40, M
|
Traumatism
|
70 days
|
Reproducible movement
to command
|
Visual fixation
|
Localization to pain
|
None
|
None
|
Without stimulation
|
|
MCS 16
|
50, M
|
ARCA
|
7 months
|
Reproducible movement
to command
|
Object localization
|
Automatic motor reaction
|
Intelligible vocalization
|
Intentional
|
Without stimulation
|
|
MCS 17
|
56, F
|
Hydrocephaly
|
75 days
|
Startle reflex
|
Visual pursuit
|
None
|
Oral reflexes
|
None
|
Without stimulation
|
|
MCS 18
|
63, F
|
Stroke
|
17 days
|
Consistent movement
to command
|
Visual fixation
|
None
|
None
|
None
|
With stimulation
|
|
MCS 19
|
17, M
|
Traumatism
|
4 months
|
Reproducible movement
to command
|
Visual fixation
|
Localization to pain
|
Oral reflexes
|
None
|
Without stimulation
|
|
MCS 20
|
32, F
|
Anoxia
|
15 months
|
Startle reflex
|
Visual pursuit
|
Abnormal posturing
to pain
|
Oral reflexes
|
None
|
With stimulation
|
|
MCS 21
|
50, M
|
Anoxia
|
85 months
|
Reproducible movement
to command
|
Object localization
|
Automatic motor reaction
|
None
|
Intentional
|
With stimulation
|
|
MCS 22
|
23, M
|
Traumatism
|
11 months
|
Reproducible movement
to command
|
Visual pursuit
|
Flexion
to pain
|
Oral reflexes
|
None
|
Without stimulation
|
|
MCS 23
|
22, M
|
Traumatism
|
99 months
|
Startle reflex
|
Visual fixation
|
Automatic motor reaction
|
None
|
None
|
Without stimulation
|
|
MCS 24
|
27, M
|
Traumatism
|
4 months
|
Reproducible movement
to command
|
Visual pursuit
|
Flexion
to pain
|
Oral reflexes
|
None
|
Without stimulation
|
|
MCS 25
|
30, M
|
Traumatism
|
131 months
|
Reproducible movement
to command
|
Visual pursuit
|
Flexion
to pain
|
Oral reflexes
|
None
|
With stimulation
|
|
MCS 26
|
36, M
|
Traumatism
|
4 months
|
Reproducible movement
to command
|
Visual pursuit
|
Flexion
to pain
|
Oral reflexes
|
None
|
Without stimulation
|
|
MCS 27
|
65, M
|
Traumatism
|
21 months
|
Reproducible movement
to command
|
None
|
Abnormal posturing
to pain
|
Vocalization
|
None
|
With stimulation
|
|
Table I. Condt.
|
|
State
|
Age, sex
|
Etiology
|
Time of PET
|
Audition
|
Visual
|
Motor
|
Verbal
|
Comm
|
Arousal
|
|
MCS 5
|
33, M
|
ARCA
|
39.5 months
|
Startle reflex
|
Visual pursuit
|
Automatic motor reaction
|
Vocalization
|
None
|
Without stimulation
|
|
MCS 6
|
64, M
|
Aneurysm
|
6 months
|
Consistent movement
to command
|
Object recognition
|
Automatic motor reaction
|
Intelligible verbalization
|
Intentional
|
With stimulation
|
|
MCS 7
|
50, F
|
Aneurysm
|
28 days
|
Startle reflex
|
Visual pursuit
|
Flexion to pain
|
Oral reflexes
|
None
|
Without stimulation
|
|
MCS 8
|
38, M
|
Anoxia
|
4 months
|
Reproducible movement
to command
|
Visual pursuit
|
Flexion
to pain
|
Oral reflexes
|
None
|
Without stimulation
|
|
MCS 9
|
81, M
|
meningitis encephalopathy
|
46 days
|
Localization ton sound
|
Visual pursuit
|
Localization to pain
|
Oral reflexes
|
None
|
Without stimulation
|
|
MCS 10
|
19, F
|
Traumatism
|
30 months
|
Startle reflex
|
Visual pursuit
|
Flexion
to pain
|
Vocalization
|
None
|
Without stimulation
|
|
MCS 11
|
46, M
|
Traumatism
|
17 months
|
Startle reflex
|
Visual pursuit
|
Flexion
to pain
|
None
|
None
|
Without stimulation
|
|
MCS 12
|
36, M
|
Traumatism
|
270 months
|
Reproducible movement
to command
|
Visual pursuit
|
Automatic motor reaction
|
None
|
None
|
Without stimulation
|
|
MCS 13
|
29, M
|
Traumatism
|
46 days
|
Startle reflex
|
Visual pursuit
|
Flexion
to pain
|
Oral reflexes
|
None
|
Without stimulation
|
|
MCS 14
|
50, F
|
ARCA
|
65 days
|
Reproducible movement
to command
|
Blink
to threat
|
Flexion
to pain
|
Vocalization
|
None
|
With stimulation
|
|
MCS 15
|
40, M
|
Traumatism
|
70 days
|
Reproducible movement
to command
|
Visual fixation
|
Localization to pain
|
None
|
None
|
Without stimulation
|
|
MCS 16
|
50, M
|
ARCA
|
7 months
|
Reproducible movement
to command
|
Object localization
|
Automatic motor reaction
|
Intelligible vocalization
|
Intentional
|
Without stimulation
|
|
MCS 17
|
56, F
|
Hydrocephaly
|
75 days
|
Startle reflex
|
Visual pursuit
|
None
|
Oral reflexes
|
None
|
Without stimulation
|
|
MCS 18
|
63, F
|
Stroke
|
17 days
|
Consistent movement
to command
|
Visual fixation
|
None
|
None
|
None
|
With stimulation
|
|
MCS 19
|
17, M
|
Traumatism
|
4 months
|
Reproducible movement
to command
|
Visual fixation
|
Localization to pain
|
Oral reflexes
|
None
|
Without stimulation
|
|
MCS 20
|
32, F
|
Anoxia
|
15 months
|
Startle reflex
|
Visual pursuit
|
Abnormal posturing
to pain
|
Oral reflexes
|
None
|
With stimulation
|
|
MCS 21
|
50, M
|
Anoxia
|
85 months
|
Reproducible movement
to command
|
Object localization
|
Automatic motor reaction
|
None
|
Intentional
|
With stimulation
|
|
MCS 22
|
23, M
|
Traumatism
|
11 months
|
Reproducible movement
to command
|
Visual pursuit
|
Flexion
to pain
|
Oral reflexes
|
None
|
Without stimulation
|
|
MCS 23
|
22, M
|
Traumatism
|
99 months
|
Startle reflex
|
Visual fixation
|
Automatic motor reaction
|
None
|
None
|
Without stimulation
|
|
MCS 24
|
27, M
|
Traumatism
|
4 months
|
Reproducible movement
to command
|
Visual pursuit
|
Flexion
to pain
|
Oral reflexes
|
None
|
Without stimulation
|
|
MCS 25
|
30, M
|
Traumatism
|
131 months
|
Reproducible movement
to command
|
Visual pursuit
|
Flexion
to pain
|
Oral reflexes
|
None
|
With stimulation
|
|
MCS 26
|
36, M
|
Traumatism
|
4 months
|
Reproducible movement
to command
|
Visual pursuit
|
Flexion
to pain
|
Oral reflexes
|
None
|
Without stimulation
|
|
MCS 27
|
65, M
|
Traumatism
|
21 months
|
Reproducible movement
to command
|
None
|
Abnormal posturing
to pain
|
Vocalization
|
None
|
With stimulation
|
|
Table I. Condt.
|
|
State
|
Age, sex
|
Etiology
|
Time of PET
|
Audition
|
Visual
|
Motor
|
Verbal
|
Comm
|
Arousal
|
|
MCS 28
|
23, M
|
Traumatism
|
73 months
|
Consistent movement
to command
|
Object recognition
|
Automatic motor reaction
|
Intelligible vocalization
|
Intentional
|
Attention
|
|
EMCS 1
|
38, M
|
ARCA
|
45 months
|
Consistent movement
to command
|
Object recognition
|
Automatic motor reaction
|
Intelligible vocalization
|
Functional
|
Attention
|
|
EMCS 2
|
45, F
|
Traumatism
|
6 months
|
Consistent movement
to command
|
Object recognition
|
Automatic motor reaction
|
Intelligible vocalization
|
Functional
|
Attention
|
|
EMCS 3
|
32, M
|
Traumatism
|
26 months
|
Consistent movement
to command
|
Object recognition
|
Functional use of object
|
Intelligible vocalization
|
Functional
|
Attention
|
|
EMCS 4
|
37, M
|
ARCA
|
9 months
|
Consistent movement
to command
|
Object recognition
|
Functional use of object
|
Intelligible vocalization
|
Functional
|
Attention
|
|
EMCS 5
|
14, M
|
Traumatism
|
14 months
|
Consistent movement
to command
|
Object recognition
|
Functional use of object
|
Intelligible vocalization
|
Functional
|
Attention
|
|
EMCS 6
|
56, M
|
Stroke
|
64 days
|
Consistent movement
to command
|
Object localization
|
Functional use of object
|
Intelligible vocalization
|
Intentional
|
Attention
|
|
EMCS 7
|
25, M
|
Traumatism
|
9 months
|
Consistent movement
to command
|
Object recognition
|
Functional use of object
|
Intelligible vocalization
|
Functional
|
Attention
|
|
EMCS 8
|
44, M
|
Stroke
|
7.5 months
|
Consistent movement
to command
|
Object recognition
|
Functional use of object
|
Intelligible vocalization
|
Functional
|
Attention
|
|
EMCS 9
|
44, M
|
ARCA
|
88 days
|
Consistent movement
to command
|
Object recognition
|
Automatic motor reaction
|
Intelligible vocalization
|
Functional
|
Attention
|
|
EMCS 10
|
76, F
|
Intoxication
|
81 days
|
Reproducible movement
to command
|
Object recognition
|
Automatic motor reaction
|
Intelligible vocalization
|
Functional
|
Attention
|
|
LIS 1
|
53, M
|
Basilar stroke
|
81 days
|
Reproducible movement
to command
|
Visual pursuit
|
Abnormal posturing to pain
|
Vocalization
|
Intentional
|
None
|
|
LIS 2
|
47, F
|
Basilar stroke
|
20 days
|
Reproducible movement
to command
|
Object recognition
|
Flexion to pain
|
Oral reflexes
|
Intentional
|
Without stimulation
|
|
LIS 3
|
39, M
|
Traumatism
|
51 months
|
Reproducible movement
to command
|
Object recognition
|
Flexion to pain
|
Oral reflexes
|
Intentional
|
Attention
|
|
LIS 4
|
44, F
|
Basilar stroke
|
52 months
|
Consistent movement
to command
|
Object recognition
|
Functional use of object
|
None
|
Functional
|
Attention
|
|
LIS 5
|
44, F
|
Basilar stroke
|
19 days
|
Consistent movement
to command
|
Object recognition
|
Flexion
to pain
|
Oral reflexes
|
Functional
|
Attention
|
|
LIS 6
|
22, F
|
Basilar stroke
|
14 days
|
None
|
None
|
Flexion
to pain
|
Oral reflexes
|
None
|
None
|
|
LIS 7
|
27, F
|
Basilar stroke
|
71 months
|
Consistent movement
to command
|
Object recognition
|
Functional use of object
|
Intelligible vocalization
|
Functional
|
Attention
|
|
LIS 8
|
42, F
|
Brain stem haemorrhage
|
56 days
|
Reproducible movement
to command
|
Visual pursuit
|
Flexion
to pain
|
None
|
Intentional
|
With stimulation
|
|
PET: positron emission tomography; VS/UWS: vegetative state/unresponsive wakefulness syndrome; MCS: minimally conscious state: EMCS: emergence from MCS: LIS: locked-in syndrome; M; male; F: female; Comm: communication; ARCA: cardiac arrest.
|
VS/UWS patients showed metabolic dysfunction in both thalami and in a widespread cortical network encompassing the extrinsic/lateral network (i.e. bilateral posterior parietal and prefrontal areas) and the intrinsic/medial network (i.e. the precuneus and adjacent posterior cingulate cortex and mesiofrontal and adjacent anterior cingulate cortex), compared with controls (Fig. 1). MCS patients showed metabolic dysfunction in both thalami and in the intrinsic/medial network. EMCS patients showed metabolic dysfunction in the posterior cingulate cortex and adjacent retrosplenial cortex. LIS patients showed metabolic dysfunction only in infratentorial regions (i.e. the cerebellum) (Table II).
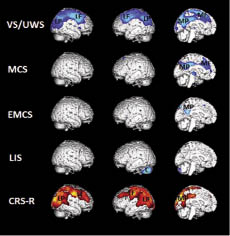
Fig. 1. Areas with significant metabolic impairment (blue) in vegetative state/unresponsive wakefulness syndrome (VS/UWS, n = 24), minimally conscious state (MCS, n = 28), emergence from MCS (EMCS, n = 10) and locked-in syndrome (LIS, n = 8) compared with age-matched controls (n = 39) (thresholded at p < 0.01 family-wise correction for multiple comparisons). The lower panel shows the areas where metabolic activity correlated with Coma Recovery Scale-Revised (CRS-R) scores (thresholded at uncorrected p < 0.001; red). Note that in VS/UWS there is a metabolic dysfunction in the thalamus (T) external network encompassing left and right lateral parietal (LP) and lateral prefrontal (LF) cortices and in the internal network encompassing midline precuneus/posterior cingulate (MP) and mesiofrontal/anterior cingulate (MF) cortices. In MCS the thalamus (T) and intrinsic network is impaired (MP, MF). EMCS shows partly impaired intrinsic network activity (MP) and LIS fully preserved awareness networks, with only impairment in the cerebellum (C). The behavioural assessment scores correlate with activity in the extrinsic network (LP, LF) and part of the intrinsic network (MP).
|
Table II. Coordinates of peak voxels of hypometabolic areas in vegetative state/unresponsive wakefulness syndrome (VS/UWS), minimally conscious state (MCS), emergence from MCS (EMCS) and locked-in syndrome (LIS)
|
|
Areas
|
x (mm)
|
y (mm)
|
z (mm)
|
Z
|
p
|
|
VS/UWS
|
|
|
|
|
|
|
Right thalamus
|
8
|
–18
|
4
|
5.21
|
< 0.0001
|
|
Left thalamus
|
–2
|
16
|
2
|
4.94
|
< 0.0001
|
|
Right lateral parietal
|
50
|
18
|
0
|
4.5
|
< 0.0001
|
|
Left lateral parietal
|
–38
|
–72
|
42
|
7.29
|
< 0.0001
|
|
Right lateral prefrontal
|
52
|
–4
|
52
|
Inf
|
< 0.0001
|
|
Left lateral prefrontal
|
–34
|
4
|
54
|
7.56
|
< 0.0001
|
|
Precuneus/posterior cingulate
|
2
|
–36
|
34
|
Inf
|
< 0.0001
|
|
Mesiofrontal/anterior cingulate
|
2
|
–36
|
34
|
Inf
|
< 0.0001
|
|
MCS
|
|
|
|
|
|
|
Right thalamus
|
4
|
–18
|
2
|
7.37
|
< 0.0001
|
|
Left thalamus
|
–4
|
–20
|
2
|
4.2
|
< 0.0001
|
|
Precuneus/posterior cingulate
|
0
|
–36
|
32
|
Inf
|
< 0.0001
|
|
Mesiofrontal/anterior cingulate
|
6
|
18
|
30
|
6.22
|
< 0.0001
|
|
EMCS
|
|
|
|
|
|
|
Posterior cingulate/restrosplenial
|
–2
|
–48
|
22
|
5.49
|
< 0.0001
|
|
LIS
|
|
|
|
|
|
|
Cerebellum
|
–38
|
–68
|
–38
|
3.88
|
< 0.0001
|
|
Inf: inferior than 0.0001.
|
At the group level, CRS-R total scores showed a positive correlation with a widespread cortical network encompassing both extrinsic/lateral network (i.e. bilateral posterior parietal and prefrontal areas) and part of the intrinsic/medial network (i.e. the precuneus and adjacent posterior cingulate cortex) (see Table III).
|
Table III. Coordinates of peak voxels from areas showing a linear positive correlation with Coma Recovery Scale-Revised total scores
|
|
Regions
|
x (mm)
|
y (mm)
|
z (mm)
|
Z
|
p
|
|
Right lateral parietal
|
50
|
18
|
0
|
4.5
|
<0.0001
|
|
Left lateral parietal
|
–58
|
–50
|
38
|
4.85
|
<0.0001
|
|
Right lateral prefrontal
|
52
|
–4
|
52
|
Inf
|
<0.0001
|
|
Left lateral prefrontal
|
–34
|
4
|
54
|
7.56
|
<0.0001
|
|
Precuneus/posterior cingulate
|
2
|
–36
|
34
|
Inf
|
<0.0001
|
|
Inf: inferior than 0.0001.
|
DISCUSSION
Our results in VS/UWS of different aetiologies show a widespread fronto-parietal cortical dysfunction, in agreement with previous studies (9, 28–30). We observed a hypometabolism in the external network encompassing left and right lateral parietal and lateral prefrontal cortices and in the internal network encompassing midline precuneus/posterior cingulate and mesiofrontal/anterior cingulate cortices. In MCS patients it seems that the extrinsic/lateral network is less impaired than is the intrinsic/medial network. This result is consistent with the clinical finding that these patients show evidence of external/sensory awareness, known to depend upon the functional integrity of the extrinsic/lateral fronto-parietal system (3, 31–35). The predominance of intrinsic/midline network impairment in MCS could reflect an impaired internal/self-awareness in these patients, which is very difficult to quantify at the bedside. Indeed, CRS-R assessments only have one item possibly assessing some form of internal/self-awareness: visual pursuit in response to a moving mirror (36).
In our view, the current data could shed some light on impaired internal/self-awareness in MCS via the study of patients’ residual brain function. An increasing body of evidence points to the critical role of the intrinsic network in the emergence of internal/self-awareness including stimulus-independent cognitive processes, such as daydreaming, mental imagery, inner speech and self-oriented thoughts (37–40). In fMRI studies, the latter network, recorded during the so-called “resting state” condition has also been coined “default mode network” (41–43). In both VS/UWS and MCS patients a significant thalamic metabolic impairment was identified, in line with previous PET (29, 30, 44) and diffusion tensor imaging (45) MRI studies, and post-mortem neuropathology (46). This finding can also be related to the clinical observation that both patient groups have fluctuating arousal levels. Indeed, in our cohort 10 out of 24 (42%) VS/UWS and 7 out of 28 (25%) MCS showed CRS-R arousal subscores of 1, meaning that patients needed tactile or noxious stimulation at least once during the examination in order to obtain sustained eye opening (47).
EMCS patients showed a near-normal brain metabolism with preserved extrinsic network activity and only dysfunction of posterior cingulate cortex and adjacent retrosplenial cortex. This area, part of the intrinsic network, is known to be involved in autobiographical memory and self-reflexion (48, 49). Clinically, EMCS patients indeed classically experience confusion and amnesia syndromes (50, 51). Finally, our studied LIS patients failed to show metabolic dysfunction in any supratentorial brain area. Both the extrinsic and intrinsic network activity was preserved in LIS and only the cerebellum was shown to be impaired, in line with previous studies (52, 53). Previous neuropsychological studies have indeed shown that classical LIS patients have no deficit in cognitive functioning (54). Despite the fact that 6/8 LIS patients experienced basilar artery stroke and showed structural lesions on MRI in the ventral pontine region (encompassing the corticospinal and adjacent corticobulbar pathways) the resulting metabolic impairment was localized not in the brainstem, but in the cerebellum. This can be explained by the fact that PET-FDG functional imaging, in contrast to MRI structural imaging, does not show white matter structural damage (i.e. in brainstem), but rather the cortical metabolic consequences (i.e. in cerebellar hemispheres), reflecting de-afferentation.
The observed progressive recovery of intrinsic network metabolic activity, as measured by FDG-PET in severely brain-damaged patients, ranging from VS/UWS, MCS, EMCS to LIS, corroborates previous fMRI “resting state” studies showing a progressive recovery of functional connectivity in the “default mode network” in these patients (55). The latter study also identified a linear correlation between CRS-R total scores and functional connectivity in the default mode network. We expand these findings here, showing an additional correlation with the extrinsic/lateral network metabolic activity and CRS-R total scores.
In conclusion, the objective measurement of extrinsic/lateral and intrinsic/midline metabolic activity in severely brain-injured patients following coma, permits us to better understand the residual external/sensory and internal/self-awareness in disorders of consciousness. Our data show, for the first time, that patients with MCS, in contrast to those with VS/UWS, show cortical dysfunction of the intrinsic/internal awareness system more than of the extrinsic/external awareness networks. If confirmed, these findings indicate an impairment of a clinically barely measurable dysfunction of internal or self-awareness in MCS.
ACKNOWLEDGEMENTS
This study was supported by the Fonds de la Recherche Scientifique (FRS), Fonds pour la Recherche Industrielle et Agronomique (FRIA), French Speaking Community Concerted Research Action, University and University Hospital of Liège, James S. McDonnell Foundation, Mind Science Foundation and European Commission (Mindbridge, DISCOS, DECODER & COST).
REFERENCES




