OBJECTIVE: To determine the long-term course of social activity after a stroke.
DESIGN: Prospective cohort study.
Patients: Patients with a first-ever supratentorial stroke were selected in 4 Dutch rehabilitation centres.
METHODS: Social activity was measured by the Frenchay Activities Index (FAI) at 1 and 3 years post-stroke to determine social activity. Changes in FAI scores ≥ 7 points were considered real change.
RESULTS: Data from 190 patients were available for analysis. The mean FAI score was stable between 1 and 3 years post-stroke. A decline in social activity was seen in 12% of all individuals and improvement in another 12%. Inactivity at 1 year post-stroke was strongly associated with inactivity at 3 years post-stroke (odds ratio (OR) = 19.9; 95% confidence interval (CI) 9.1–43.3). Motor impairment of the leg (OR = 0.39; 95% CI 0.15–0.97) and being socially inactive at 1 year post-stroke (OR = 0.19; 95% CI 0.04–0.84) were associated with a lower risk of decline in FAI scores.
CONCLUSION: For the majority of stroke patients, the level of social activity is stable during the chronic phase (beyond 1 year post-stroke). Only 1 in 10 patients showed improvement, and 1 in 10 declined. The level of social activity at 1 year post-stroke is indicative of the level of social activity at 3 years post-stroke. Rehabilitation professionals should focus their follow-up programmes on patients inactive at 1 year post-stroke, as this group is at risk for chronic inactivity, and should be stimulated to achieve social reintegration.
Key words: participation; longitudinal studies; prognosis; course; long-term care; cerebrovascular accident; social activity.
J Rehabil Med 2012; 00: 00–00
Correspondence address: V. P. M. Schepers, Department of Rehabilitation, Nursing Science and Sports Medicine, University Medical Center Utrecht, PO Box 85500, NL-3508 GA Utrecht, The Netherlands. E-mail: V.P.M.Schepers-3@umcutrecht.nl
Submitted December 7, 2010; accepted September 19, 2011
Introduction
Stroke is the most disabling chronic disease in Europe (1), with a high risk of diminished levels of social activity (2). Social activity includes activities of family, work and leisure (3). Achieving optimal social reintegration is a major goal in stroke rehabilitation. Knowledge about the process of recovery of social activity is very important to patients, relatives and rehabilitation professionals. According to some studies, patients show an increase in social activity in the first year after a stroke (4–8). In an earlier article (3) we showed that it is possible to predict, even at the start of the inpatient rehabilitation programme, which patients are at risk for social inactivity one year post-stroke. The predictors we found were age, gender, living with a partner, level of motor impairment, level of dependency in activities of daily living (ADL) and communication. Little evidence is available on the course of social activity beyond one year post-stroke. Three longitudinal studies (9–11) found that the mean course of social activity was stable in the long term. However, these studies did not evaluate individual changes in social activity. Only one study (11) determined individual changes in social reintegration in the long term, i.e. between 1 and 3 years post-stroke, using the London Handicap Scale (LHS). Across the different items of the LHS, they found a decline of one or more levels in 19–33% of the patients, and improvement in 22–39%.
Thus, evidence about the long-term course of social activity in individual stroke patients is still limited. In current clinical practice, the follow-up of most stroke patients by rehabilitation professionals will end approximately one year post-stroke. However, some patients may improve thereafter, by adapting to the chronic situation, while others may decline, due to problems such as deteriorating mobility. Knowledge of the course of social activity beyond one year post-stroke could help healthcare workers to determine which stroke patients require continued follow-up and rehabilitation care.
The aims of this study were: (i) to determine the mean and individual course of social activity between 1 and 3 years after a stroke; and (ii) to find predictors of decline in social activity. Candidate predictors were selected based on earlier studies. (3)
Methods
Participants
Subjects were selected from stroke patients consecutively admitted to 4 rehabilitation centres in the Netherlands, and were invited to participate in the Functional Prognosis after Stroke (FuPro-Stroke) study (3). Inclusion criteria were first-ever stroke, one-sided supratentorial lesion and age above 18 years. Exclusion criteria were disabling co-morbidity (pre-stroke Barthel Index (BI) below 18) and inability to speak Dutch.
Procedure
The study was approved by the medical ethics committees of the University Medical Centre Utrecht and the participating rehabilitation centres. Informed consent was obtained from all patients. If a patient had communication problems, both the patient and a proxy gave informed consent. The Frenchay Activities Index (FAI) was assessed at 1 (T1) and 3 (T2) years after stroke in all patients living at home at the time of the assessment. If a patient had communication problems, the proxy answered the FAI questions. All assessments were carried out by trained research assistants.
Measures
Social activity was assessed using the FAI (12), which consists of 15 items measuring complex activities in the categories of household, recreation, transportation and work. The FAI is specifically developed as an outcome measure of stroke rehabilitation. It is widely used for the assessment of social activity in stroke research. We used the Dutch translated version of the FAI (13). The FAI item score is based on the frequency with which an activity was performed, and ranges from 0 (low frequency) to 3 (high frequency). Ten items concern the past 3 months and 5 items concern the past 6 months. The FAI total score is the sum of item scores, and ranges from 0 (inactive) to 45 (highly active). The FAI is considered a valid (13, 14) and reliable (13–16) instrument. The questions can be answered by patients or by proxies (17). Subjects were categorized as in previous studies (8, 9): inactive (range 0–15), moderately active (range 16–30) and highly active (range 31–45).
Data on demographic variables were derived from medical charts. ADL dependency was measured by the BI (18). The total BI score (0–20) was dichotomized into “dependent” (BI < 19) and “independent” (BI 19 or 20). The Motricity Index (MI) (19) was used to determine the motor functions of the arm (MI arm) and leg (MI leg). The MI scores (0–100) were dichotomized and scores lower than 75 for MI leg and lower than 76 for MI arm indicated impaired motor function. Neuropsychological status was assessed using tests for cognition and aphasia, including the Mini Mental State Examination (MMSE) (20), the short version of the Token Test (21) and the Utrecht Communication Observation (UCO) (22). Patients who had an MMSE ≤ 23 or who were aphasic (scoring > 9 on the Token Test or < 4 on the UCO) were considered to have neuropsychological impairment.
Statistical analysis
Data were analysed with SPSS version 15.0, using only data of patients with complete FAI scores for both measurements. We checked for selective non-response using χ2 analyses, independent samples t-tests or Mann-Whitney U test on the baseline characteristics of the patients who were and were not available for analyses. Total FAI scores at 1 and 3 years after stroke were compared by means of the paired t-test. The association between the total FAI scores at T1 and T2 was determined as Pearson correlation coefficients. The FAI item scores at 1 and 3 years post-stroke were compared using the Wilcoxon signed-rank test. To reduce the risk of chance findings, a p-value of 0.003 (0.05 divided by 15 FAI items) was considered statistically significant in these item analyses. In the evaluation of the individual course of the FAI, a change of ≥ 7 points on the FAI was considered a real change on statistical level (15, 16, 23). A univariate logistic regression analysis was conducted to identify statistically significant predictors of decline (≥ 7 points decline against the rest) of social activity. The candidate predictors were: gender, age above 55 years, marital status, pre-stroke employment status, comorbidity, type of stroke, hemisphere, impaired MI arm and leg, neuropsychological impairment, ADL dependency and being socially inactive at T1. Because of the few patients with real decline (n = 22), no multivariate analysis could be conducted.
Results
At the start of inpatient rehabilitation 308 patients were included for the FuPro-Stroke study. Of these 8 died, 15 had a recurrent stroke, 18 refused and 3 were lost to follow-up. A total of 264 patients were assessed at 1 year post-stroke. During follow-up, 13 patients died, 33 withdrew and 13 patients were otherwise lost to follow-up. A total of 205 patients were thus assessed 3 years post-stroke. Complete FAI data for both 1 and 3 years post-stroke were available for 190 patients. As we checked for selective non-response baseline characteristics of patients whose data were complete and available for analysis were significantly different from those whose data were not available for analyses, regarding MI total, neuropsychological impairment and BI (Table I).
| Table I. Characteristics of stroke patients at 1 year after stroke |
| | Available for analysis (n = 190) | Not available for analysis. (n = 74) |
| Gender, % female | 41.1 | 33.8 |
| Age, years, mean (SD) | 55.8 (10.5) | 57.6 (11.8) |
| % ≥ 55 years | 54.2 | 64.9 |
| Partner, % with partner | 74.7 | 78.4 |
| Pre-stroke employment status, % employed | 44.2 | 40.5 |
| Co-morbidity, % with co-morbidity | 53.7 | 63.5 |
| Type of stroke, % infarction | 71.1 | 79.7 |
| Hemisphere, % right | 47.1 | 44.6 |
| MI arm, % impaired | 51.6 | 71.6* |
| MI leg, % impaired | 56.9 | 74.3* |
| Neuropsychological, % impairment | 24.7 | 51.4* |
| BI, % dependent | 36.0 | 50.0 |
| *p < 0.05. SD: standard deviation; MI: Motricity Index; BI: Barthel Index. |
There was no significant change in mean total FAI scores between T1 (19.5, standard deviation (SD) 9.3) and T2 (19.5, SD 9.5), t = 0.02, p = 0.98. Both scores were strongly correlated (r = 0.81, p < 0.005).
The FAI item scores at 1 year and 3 years post-stroke are displayed in Table II. There was a significant decline in the percentage of patients engaging in “social outings” and “walking outdoors”. “Driving/train travel” showed significant improvement.
| Table II. Frenchay Activities Index (FAI) item scores at 1 and 3 years after stroke (n = 190) |
| FAI item | Low score (0–1) |
| 1 year post-stroke | 3 years post-stroke |
| Domestic | | |
| 1. Preparing meals | 54.2 | 53.7 |
| 2. Washing up | 37.3 | 31.6 |
| 3. Washing clothes | 58.9 | 57.4 |
| 4. Light housework | 47.9 | 53.2 |
| 5. Heavy housework | 63.7 | 70.5 |
| Leisure/work | | |
| 7. Social outings | 15.2 | 21.6* |
| 9. Pursuing hobby | 31.6 | 22.6 |
| 13. House/car maintenance | 85.3 | 84.2 |
| 15. Gainful work | 93.1 | 90.5 |
| Outdoor | | |
| 6. Local shopping | 26.8 | 36.3 |
| 8. Walking outdoors | 32.6 | 48.9* |
| 10. Driving/train travel | 63.7 | 55.3* |
| 11. Outings/car rides | 64.2 | 54.7 |
| 12. Gardening | 75.3 | 74.2 |
| 14. Reading books | 72.1 | 72.6 |
| Range of item scores = 0 (lowest frequency) to 3 (highest frequency). * = p ≤ 0.003 in Wilcoxon signed-rank test (alpha = 0.05/13 to correct for multiple testing). |
Individual course
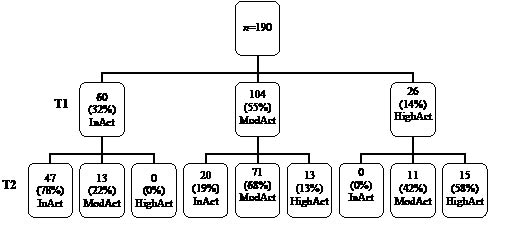
Fig. 1 shows the distribution of patients over the FAI categories (inactive, moderately active and highly active) at T1 and T2. More than half of the patients were moderately active at T1. There were no inactive patients at T1 who had become highly active at T2, nor highly active patients at T1 who had become inactive at T2. Of the 60 patients who were inactive at T1, 78% were also inactive at T2. Inactivity at T1 was strongly associated with inactivity at T2, with an odds ratio of 19.9 (95% CI 9.1–43.3).
Fig. 1. Distribution of patients over the Frenchay Activities Index (FAI) categories at T1 (1 year after stroke) and T2 (3 years after stroke). InAct: inactive; ModAct: moderately active; HighAct: highly active.
A decline (≥ 7 points) in social activity between T1 and T2 was found in 22 patients (11.6%), whereas 145 patients (76.3%) had maintained their level of social activity and 23 patients (12.1%) showed improvement between 1 and 3 years after the stroke.
The univariate analysis showed that only impaired MI leg (OR = 0.39; 95% CI 0.15–0.97) and being socially inactive at T1 (OR = 0.19; 95% CI 0.04–0.84) were associated with a significantly lower risk of FAI decline. No other predictors of FAI decline were found.
Discussion
The mean course of social activity was stable between 1 and 3 years post-stroke, and the same was true for the course of most individual patients. Only 1 in 10 patients showed improvement, while 1 in 10 declined. For the large majority of patients, the level of social activity at 1 year post-stroke is indicative of the level of activity at 3 years post-stroke. Motor impairment of the leg and being socially inactive at 1 year post-stroke were weakly associated with a lower risk of a decline in social activity.
The mean FAI total score at 1 year post-stroke, 19.5, was within the range of 12–21 found in previous studies (6, 7, 13, 24, 25). Like other studies (9–11), we found a stable mean course of social activity beyond 1 year post-stroke. The prevalence of inactivity we found was similar to the findings of another rehabilitation-based study (26) at 3 years post-stroke. However, a community-based stroke study (9) reported higher proportions, respectively 55% and 51% of the patients, being inactive at 1 and 3 years post-stroke, respectively, against 32% and 35% in our study group.
At item level, we found a significant decline in social outings and walking outdoors. This decline probably reflects the deteriorating mobility which is seen in a proportion of the stroke population in the longer term (27). The other significant change at item level was an increase in the frequency of car driving and train travel. This is largely the consequence of Dutch legislation, which is such that it often takes a long time after the stroke to obtain official permission to drive a car again.
The longitudinal design of our study made it possible to consider the course of social activity of individual patients. Either decline or improvement was found in 12% of the patients; the remaining 76% had maintained their level of social activity. Compared with Harwood et al’s study (11), which also evaluated the individual course, we found lower percentages of change. This discrepancy could be explained by a difference in measurement instrument or a difference in definition of change. In our study, we defined change as a difference of at least 7 points on the FAI, thus considering only change beyond chance. In the study by Harwood et al. (11), all change (including change caused by measurement error) on the LHS was considered real change, which may have resulted in a larger proportion of persons being recorded as showing change. The small number of predictors of decline found in our study might be the result of the small number of patients who showed a real decline. Patients who were socially inactive, as measured by the FAI, at 1 year post-stroke or had a motor impairment of the leg had a lower risk on a decline in FAI score. It is not very surprising that patients who already have a low FAI score (0–15) are less likely to show a further decline of 7 points or more. The relationship between a decline in FAI score and the motor impairment of the leg might be due to the same phenomenon, as the MI and FAI are known to be associated (3).
So far, only one study (11) has been published previously about the individual course of social activity beyond one year after stroke. The strengths of our study are the larger patient group and the use of an extensively validated outcome measure that is recommended for use in stroke studies (13, 14). In interpreting our results, some limitations of the study must be considered. First, our study was carried out in a selected stroke population, namely those who received inpatient rehabilitation. Stroke patients selected for rehabilitation are relatively young, on average moderately disabled, and often employed pre-stroke, probably resulting in a more socially active lifestyle. Unfortunately, not all data from our total study population were complete and thus available for analyses. Patients whose data were available for analyses were less disabled than those whose data were unavailable, which may have resulted in overestimation of the long-term level of social activity of stroke patients. Secondly, we used the FAI to measure social activity in our study population. Although this is a valid and popular outcome measure in stroke research, it has its limitations. It measures the frequency of performance of activities and not, for example, perceived problems or fulfilment of social roles. It does not measure whether the activities are meaningful for the patients and gives no direct insight into the pre-stroke activity level. In addition, it is physically oriented and contains many items about household activities. Thirdly, we have no details on whether the patients had access to rehabilitation in the intervening time between the 1- and 3-year assessments. Finally, we were not able to perform a multivariate analysis, and only 2 of the 11 univariate analyses showed a significant result, so that there is a chance of a type I error due to multiple testing. This, however, does not affect our main conclusion.
We conclude that, for the majority of stroke patients, the level of social activity remains stable beyond 1 year post-stroke, so the level of social activity at 1 year post-stroke is indicative of the level of social functioning at 3 years post-stroke. We recommend that rehabilitation professionals focus their follow-up programmes on patients who are socially inactive at 1 year post-stroke, as this group is at risk for chronic inactivity and needs to be stimulated to achieve social reintegration.
Acknowledgements
The project was undertaken as part of the “Functional Prognostication and Disability Study on Neurological Disorders”, supervised by the Department of Rehabilitation Medicine of the VU Medical Center, Amsterdam, and supported by the Netherlands Organization for Health Research and Development (grant: 1435.0001).
We would like to thank the participating patients and rehabilitation centres: Rehabilitation Centre De Hoogstraat, Utrecht; Rehabilitation Center Amsterdam, Amsterdam; Heliomare, Wijk aan Zee; and Blixembosch, Eindhoven.
References