OBJECTIVE: Curved walking requires complex adaptations, including shift of body weight to counteract the ensuing centrifugal force, and the production of strides of different length between legs. We hypothesized that gait capacities would be more stressed in hemiparetic patients than in healthy subjects when walking along curved, compared with straight, trajectories.
METHODS: Twenty chronic, stabilized stroke patients and 20 healthy subjects walked along straight or curved trajectories. Mean cadence and gait velocity were off-line computed from video recordings. An electronic walkway detected asymmetry of single support and degree of foot yaw angle at mid-stance. Centre of pressure during standing was recorded by posturography.
RESULTS: Compared with linear walking, the velocity of curved walking was not significantly smaller in patients, and was independent of affected body side or direction of rotation. It was inversely correlated with paretic limb weakness, asymmetry of single support, and shift of centre of pressure toward the healthy side. External rotation of the paretic foot relatively favoured curved walking toward the paretic side.
CONCLUSION: Curved locomotion is defective in stabilized stroke patients, but impairment is not dependent on direction of rotation, indicating a shared task between legs or occurrence of effective functional adaptation. These findings advocate rehabilitation exercises targeting complex gait adaptations, including curved walking.
Key words: stroke; straight walking; curved walking; cadence; walking velocity.
J Rehabil Med 2010; 42: 858–865
Correspondence address: Antonio Nardone, Division of Physical and Rehabilitation Medicine, Fondazione Salvatore Maugeri (IRCCS), Veruno (NO), Italy. E-mail: antonio.nardone@fsm.it.
Submitted January 10, 2010; accepted June 3, 2010
INTRODUCTION
Walking along curved trajectories implies a different spatio-temporal pattern of muscle activation compared with straight walking (1–4). The stride length of the inner leg (the one closer to the centre of curvature of the trajectory) is necessarily shorter than that of the outer leg, while step frequency remains the same for both legs (1). In healthy subjects (HS), both feet are oriented toward the curved trajectory, although the inner foot is placed on the floor at a greater yaw angle than the outer foot with respect to the direction of the walking path. Stance phase duration is shorter for the outer than the inner leg. Ground reaction force is greater for the outer foot at heel strike and toe-off, whereas it is greater for the inner foot during the mid-stance phase (4). Trunk roll is greater towards the inner than the outer side. The body’s centre of mass is shifted inwards, thereby creating the centripetal force that keeps the body moving along the circular path. Amplitude and timing characteristics of muscle activation are significantly associated with the spatial and temporal adaptations to curvilinear locomotion (5).
Given the substantial complexity of the changes required to accomplish smooth locomotion along circular trajectories, we investigated the capacity of hemiparetic stroke patients (HP) to perform this task. HP have difficulty in changing the direction of their locomotor trajectory (6), show poor bilateral coordination (7) and reduced cortico-spinal drive to the muscles of the affected limb (8). However, there is no information as to the quality of locomotion along a circular path in HP. We hypothesized that: (i) velocity would be slower for curved than straight walking in HP, and slowing would be greater than in HS; (ii) curved-walking ability would be related to asymmetry of standing posture; and (iii) the inner or outer location of the paretic limb with respect to the trajectory and the related foot orientation at foot fall would affect curved walking.
MATERIALS AND METHODS
Participants
We studied 20 chronic HP (12 males; mean age 62.8 years; standard deviation (SD) 11.3) and 20 gender- and age-matched HS (8 males; mean age 64.6 years; SD 10.8). Inclusion criteria were: supra-tentorial ischaemic stroke; time-interval after stroke longer than one year (mean 8.8 years; SD 6.3); capacity of walking without assistance or aids. Six patients used a cane; however, they walked without it during the walking trials. Exclusion criteria were: orthopaedic or other neurological diseases; neglect; or cognitive and communication impairments. No HP was receiving anti-spastic drugs. Some patients were receiving medications known possibly to affect balance and gait, such as antidepressants, benzodiazepines and neuroleptics (9). No patient reported experiencing dizziness or drowsiness at the time of testing. The clinical findings for the HP are summarized in Table I.
| Table I. Clinical features of hemiparetic patients |
| Patients | Sex | Age (years) | Aetiology | Localization of lesion | Side of hemiparesis | Years from lesion | Modified Ashworth Hip abd/add1 | Modified Ashworth Hip flex/ext1 | Modified Ashworth Knee1 | Modified Ashworth Ankle1 | Motricity Index2 | Myotatic Reflex Scale3 | Plantar response (Norris scale)4 |
| 1 | M | 78 | H | Thalamus | R | 4 | 0 | 0 | 0 | 2 | 54 | 2.5 | 3 |
| 2 | M | 62 | I | Cerebral peduncle | R | 2 | 0 | 1 | 1 | 1 | 54 | 3.5 | 3 |
| 3 | M | 63 | I | Middle cerebral artery | L | 19 | 0 | 1 | 1 | 2 | 73 | 2.5 | 1 |
| 4 | F | 74 | H | Frontal | L | 5 | 0 | 1 | 1 | 1 | 92 | 2.5 | 3 |
| 5 | F | 60 | H | Thalamo-capsular, corona radiata | R | 21 | 0 | 0 | 0 | 2 | 72 | 3 | 3 |
| 6 | F | 59 | I | Capsular, fronto-parietal | R | 8 | 1 | 1 | 2 | 2 | 60 | 2 | 3 |
| 7 | M | 70 | H | Capsular-lenticular, corona radiata | L | 17 | 1 | 1 | 1 | 2 | 48 | 3 | 0 |
| 8 | M | 66 | H | Temporo-parietal, parasilvian | R | 19 | 0 | 0 | 1 | 1 | 64 | 2 | 3 |
| 9 | M | 83 | I | Corona radiata | L | 4 | 2 | 1 | 2 | 1 | 64 | 2 | 0 |
| 10 | F | 31 | I | Middle cerebral artery | R | 1 | 0 | 0 | 1 | 1 | 70 | 3 | 3 |
| 11 | M | 56 | I | Corona radiata | R | 5 | 1 | 0 | 1 | 0 | 73 | 2 | 3 |
| 12 | M | 65 | I | Middle cerebral artery | R | 11 | 0 | 0 | 2 | 2 | 64 | 3 | 3 |
| 13 | M | 64 | I | Capsulo-lenticular, frontal | R | 13 | 2 | 1 | 2 | 1 | 29 | 3 | 3 |
| 14 | F | 49 | I | Middle cerebral artery | R | 6 | 0 | 0 | 1 | 2 | 73 | 2.5 | 3 |
| 15 | M | 58 | I | Middle cerebral artery, thalamo-capsular | L | 12 | 1 | 1 | 2 | 2 | 54 | 1 | 0 |
| 16 | F | 70 | I | Middle cerebral artery | L | 3 | 1 | 1 | 2 | 2 | 54 | 3 | 0 |
| 17 | M | 72 | I | Middle cerebral artery | L | 5 | 1 | 1 | 2 | 2 | 76 | 1 | 1 |
| 18 | F | 65 | I | Capsular | R | 2 | 0 | 0 | 0 | 2 | 59 | 2 | 3 |
| 19 | F | 60 | I | Middle cerebral artery | R | 11 | 1 | 0 | 1 | 1 | 48 | 2 | 0 |
| 20 | M | 50 | H | Middle cerebral artery | L | 7 | 1 | 0 | 2 | 4 | 24 | 3.5 | 0 |
| Mean (SD) | 62.8 (11.3) | | | | 8.8 (6.3) | 0.6 (0.7) | 0.5 (0.5) | 1.3 (0.7) | 1.7 (0.8) | 60.3 (15.8) | 2.5 (0.7) | 1.9 (1.4) |
| Median (range) | 63.5 | | | | 6.5 | 0.5 (0–2) | 0.5 (0–1) | 1 (0–2) | 2 (0–4) | 62.0 | 2.5 | 3.0 |
| 1From 0 (no increase in muscle tone) to 5 (rigid in flexion or extension) (9). 2From 1 (no movement) to 100 (normal strength) (10). 3From 0 (reflex absent) to 4 (reflex enhanced) (11). 4From 0 (Babinski’s sign) to 3 (normal flexor response) (12). H: haemorrhagic; I: ischaemic; R: right; L: left; abd: abduction; add: adduction; flex: flexion; ext: extension; SD: standard deviation. |
All experimental procedures were conducted in accordance with the Declaration of Helsinki and carried out with the understanding and written consent of the participants and with ethical approval of the institutional review board.
Clinical characteristics of the patients
Muscle strength and tone, tendon-tap reflexes and plantar response were clinically assessed in the lower limbs. Muscle tone was graded using the Modified Ashworth Scale (MAS) (10). Table I reports the MAS score of hip, knee and ankle joints. For scoring muscle strength, the Motricity Index was used (11). The Achilles tendon-tap reflex was scored according to the myotatic reflex scale (12). The plantar response was graded according to the Norris scale (13).
Quiet stance measurements
Centre of pressure (CoP) was recorded by means of a force platform (Medicapteurs, Balma, France). HS and HP stood barefoot with eyes open (EO) and with eyes closed (EC). Feet were placed at an angle of approximately 30°, in correspondence to the footprints displayed on the upper surface of the platform. On the antero-posterior axis, the zero value on the force plate was 100 mm advanced with respect to the line joining the heels. Distance between the medial aspect of the heels was 2–3 cm. Two 51-second trials for each visual condition were performed. The forces acting on the platform were sampled at 5 Hz. A software program calculated: (i) the absolute value of the mean CoP position on the antero-posterior (A-P) and medio-lateral (M-L) axis; (ii) sway area (SA), i.e. the 95% confidence ellipse of the dispersion of CoP positions; and (iii) sway path (SP), i.e. the distance covered by the moving instantaneous CoP (14).
Linear and curved floor walking
HP and HS walked EO along linear and circular trajectories for one min, at a self-paced cadence and velocity. They performed only one walking trial for each condition to avoid tiredness: straight ahead; clockwise (CW); and counter clockwise (CCW) (15). The circular path (radius 1.5 m) was marked with pieces of light grey tape (approximately 5 cm long by 2 cm wide) stuck to a yellowish floor. The markers were placed along the virtual circumference at deliberately irregular intervals of approximately 6 markers/m. All patients walked along this same path without paying attention to the space between markers. A researcher stood at a distance that did not cause any interference. Given the relatively simplicity of the task, and in order to avoiding fatigue, only short-distance “familiarization trials” were required. No patient stopped during the trial under any condition. For further analysis, left-side affected HP (LH) or right-side affected HP (RH) were assigned to 2 groups, i.e. walking with the paretic limb inside the curvature (PI) (or turning toward the paretic side) and walking with the paretic limb outside (PO). The order of the conditions was randomized across participants. The 3 trials were performed on the same day, separated by time-intervals of at least 5 min, depending on the patient’s compliance. The lower limbs were video-recorded; length of walking trajectory and mean cadence along the straight and circular trajectories were off-line computed by visual analysis of the video-recordings of the foot falls along the marked path, replayed on the computer monitor.
Walking on the electronic walkway
On the same day, gait variables (velocity, cadence, yaw angle of feet, duration of single support) during linear walking were recorded and analysed through the GAITRite® walkway (CIR systems Inc., NY, USA). Participants began walking 2 m ahead of the 4-m mat and continued walking 2 m past its end. All walked at the same velocity used for floor walking, and performed a sequence of 4 walking trials. Prior to data collection, participants completed one walking trial in order to familiarize themselves with the procedure.
Data treatment and statistical analysis
Comparisons of the mean positions in the sagittal and medio-lateral plane, and of the area and path of CoP sway, were performed by 2-way analysis of variance (ANOVA) between groups (HS, HP) as independent variables and within visual conditions (EO, EC) as repeated-measures. For comparisons of spatial and temporal variables during floor walking, ANOVA between groups (HS, HP) and within walking conditions (straight, CW or PI, CCW or PO) was used. This analysis was repeated for the first 30 s and the second 30 s of each trial in order to assess any effect of fatigue. When ANOVA showed significant differences (p < 0.05), the Newman-Keuls post-hoc test was used. Correlation coefficients between clinical and spatio-temporal gait variables were calculated using Spearman’s rank method for ordinal variables. The results from the electronic walkway obtained during each trial were averaged for further analysis. The asymmetry in the duration of the single support was calculated using the asymmetry index = (unaffected side – affected side)/(affected side + unaffected side)*100 (16). The relationship between asymmetry index or velocity during CW and CCW walking and foot yaw angle was assessed by correlation analysis. Where appropriate, means and SD are reported in the text. In the figures, error bars for standard errors (SE) are reported. The software package Statistica® (StatSoft, Tulsa, Oklahoma, USA) was used for all analyses.
RESULTS
Quiet stance data
Table II summarizes the results and statistics. Sway path and sway area were slightly larger in HP than in HS, but significantly larger with EC than EO in both groups. There was no interaction between groups and visual conditions. The CoP M-L position lay within a few mm distance from the sagittal plane in HS; conversely, in HP, CoP was mostly shifted toward the unaffected limb, irrespective of the lesion side. On average, the CoP was approximately 20 mm more advanced in the patients than in the healthy subjects, which was in keeping with previous results (17).
| Table II. Results of stabilometric evaluations in healthy subjects and hemiparetic patients |
| | | Normal subjects Mean (SD) | Hemiparetic patients Mean (SD) | ANOVA |
| Sway path (mm) | EO | 346.1 (106.5) | 459.8 (203.1) | HS vs HP (F = 3.4, df = 1,35, p = n.s.) |
| EC | 601.6 (269.9) | 811.9 (458.6) | EO vs EC (F = 69.0, df = 1,35, p < 0.0001) |
| | | | | Interaction (F = 0.1, df = 1,35, p = n.s.) |
| Sway area (square mm) | EO | 103.2 (47.4) | 153.7 (98.1) | HS vs HP (F = 2.7, df = 1,35, p = n.s.) |
| EC | 205.8 (96.7) | 375.9 (375.8) | EO vs EC (F = 66.8, df = 1,35, p < 0.0001) |
| | | | | Interaction (F = 0.002, df = 1,35, p = n.s.) |
| CoP ML1 (mm) | EO | 6.7 (4.8) | 17.3 (14.3) | HS vs HP (F = 10.1, df = 1,35, p < 0.005) |
| EC | 5.6 (5.5) | 18.7 (15.3) | EO vs EC (F = 0.03, df = 1,35, p = n.s.) |
| | | | | Interaction (F = 2.9, d = 1,35, p = n.s.) |
| CoP AP (mm) | EO | –29.2 (17.1) | –9.7 (17.2) | HS vs HP (F = 11.7, df = 1,35, p < 0.005) |
| EC | –24.9 (14.9) | –7.8 (16.8) | EO vs EC (F = 9.6, df = 1,35, p = 0.005) |
| | | | | Interaction (F = 1.4, df = 1,35, p = n.s.) |
| Analysis of variance (ANOVA) of Sway path and Sway area has been performed on the logarithmic values. 1Absolute value of the mean. EO: eyes open; EC: eyes closed; HS: healthy subjects; HP: hemiparetic patients; n.s.: not significant; SD: standard deviation. |
Characteristics of floor walking
Walking cadence. There was a group effect on cadence (F = 45.3, df = 1,38, p < 0.001). Cadence during linear walking was approximately 25% lower in HP than HS (HP: 87.4 (SD 19.5) steps/min; HS: 116.8 (SD 6.5) steps/min). The same was true for curved walking, both for CW or PI (HP: 82.2 (SD 17.4) steps/min; HS: 111.1 (SD 9.2) steps/min) and CCW or PO (HP: 81.0 (SD 18.8) steps/min; HS: 111.2 (SD 8.5) steps/min). Walking conditions affected cadence in both HS and HP (F = 20.4, df = 2,76, p < 0.001). In both groups, cadence decreased by approximately 5% during curved walking (post-hoc, p < 0.005), featuring a non-significant interaction. No cadence difference was found between directions of curved walking in either HS or HP (F = 0.75, df = 1,38, p < 0.39).
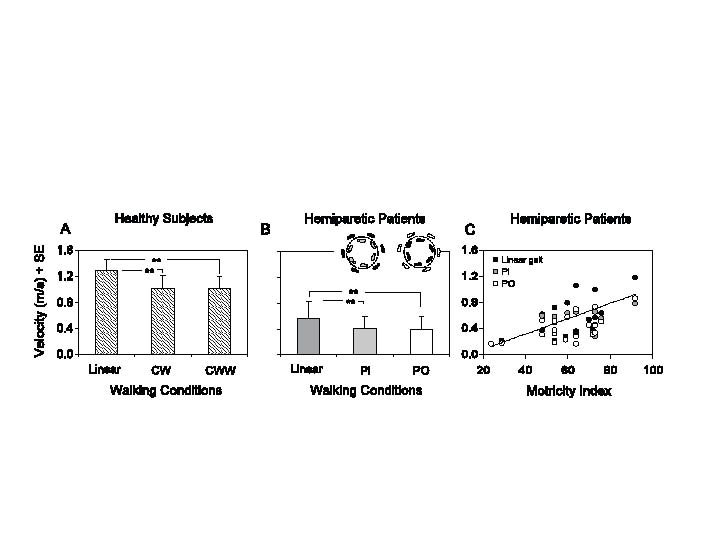
Velocity. There was a group effect on gait velocity (F = 117.8, df = 1,38, p < 0.001), since mean distances travelled by the patients were shorter, by approximately 60%, compared with HS (Fig. 1A, B). There was an effect of walking conditions (F = 117.7, df = 2,76, p < 0.001). The interaction was significant (F = 8.3, df = 2,76, p < 0.001), the changes in velocity between conditions being smaller in HP. During curved walking, the direction of rotation did not affect the average walking velocity, in either HS or HP (post-hoc test, p > 0.6 for both groups). Velocity, during either curved or linear walking, was positively correlated with paretic limb strength (Fig. 1C) (linear, y = 0.012x – 0.159, R2 = 0.33, p < 0.01; PI, y = 0.006x – 0.029, R2 = 0.31, p < 0.01; PO, y = 0.008x – 0.056, R2 = 0.35, p < 0.01).
Fig. 1. (A) Mean velocity under 3 different walking conditions in healthy subjects: along the linear, circular clockwise (CW) and counter clockwise (CCW) trajectory. (B) Mean velocity along the linear trajectory in hemiparetic stroke patients, with the paretic foot placed inside (PI) or outside the trajectory (PO). The insets show the footprints (healthy side, black; paretic side, grey or white, according to the inner or outer position of the paretic foot, respectively. (C) Relationship between gait velocity and Motricity Index across all patients during the 3 walking conditions.**p < 0.001.
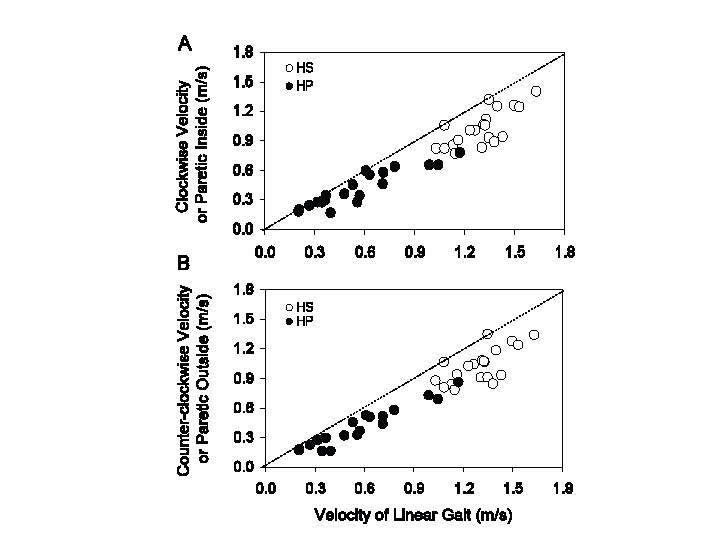
Distribution of the velocities across HS and HP. The velocity during curved walking is plotted against that during straight walking for HP and HS participants in Fig. 2. There was a positive relationship between the 2 variables, in both groups and for both directions of curved walking collapsed (HS, y = 0.84x – 0.07, p < 0.001, R2 = 0.53; HP, y = 0.62x + 0.06, p < 0.001, R2 = 0.84). On average, both HS and HP walked relatively shorter distances during circular than straight walking. In the patients, the difference curved minus straight tended to disappear for the slowest walking velocities.
Fig. 2. Relationship between curved ((A): clockwise or paretic inside; (B): counter clockwise or paretic outside) and linear walking in healthy subjects (HS) (open circles) and hemiparetic stroke patients (HP) (black circles). Walking velocities are slower in HP than HS, with minimal overlap at approximately 1 m/s. The dotted line represents the identity line. All the data-points lie below the identity line for both HS and HP, indicating slower velocity of curved walking.
Participants were not slower in the second part of the walking trials. Minimal changes were observed between the first and second half of the trials. Cadence diminished by less than 1% in both HP and HS (F = 0.3, df = 2,76, p = 0.76), regardless of the trajectory shape (all participants collapsed, F = 2.5, df = 2,76, p = 0.09). Walking velocity diminished by less than 2% in both HP and HS (F = 0.5, df = 2,76, p = 0.61), regardless of the trajectory shape (F = 0.04, df = 2,76, p = 0.97).
Temporo-spatial characteristics of the foot placement on the electronic walkway
Linear walking on the electronic walkway was comparable to linear floor walking; this was true for both cadence and velocity and for both HS and HP. Student’s t-test showed no difference between the 2 walking conditions (all subjects collapsed: cadence p = 0.25; velocity p = 0.99). The regression lines drawn across all subjects were y = 0.87x + 0.12, p < 0.001, R2 = 0.92 for velocity and y = 0.77x + 21.8, p < 0.001, R2 = 0.89 for cadence (not shown).
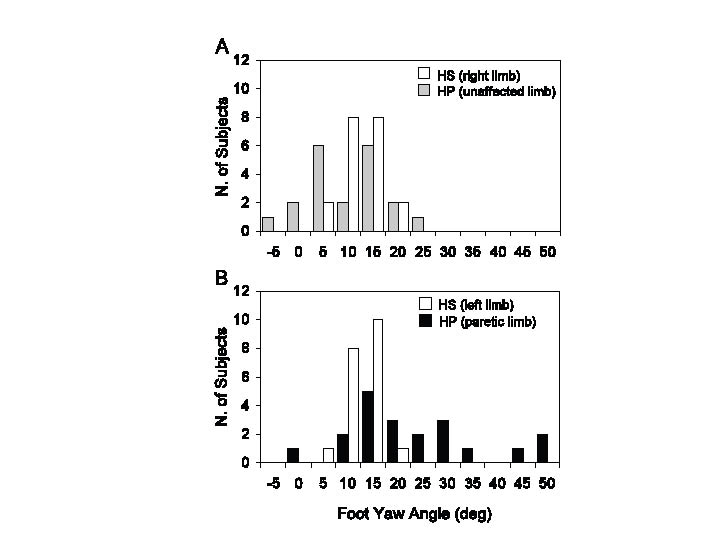
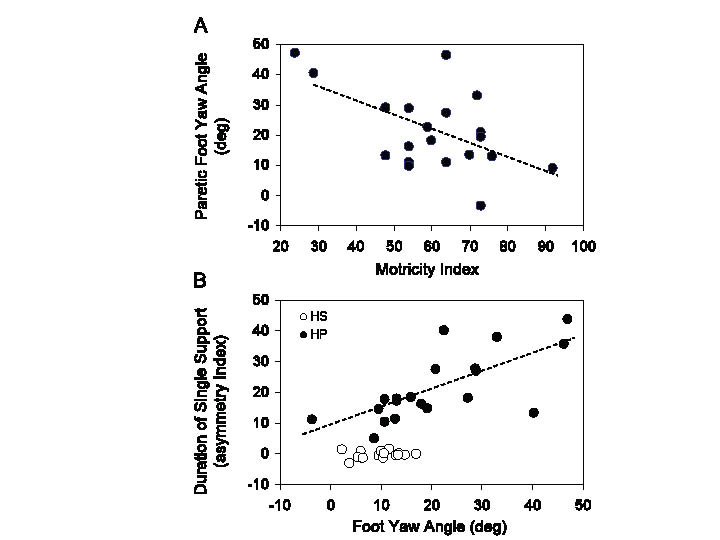
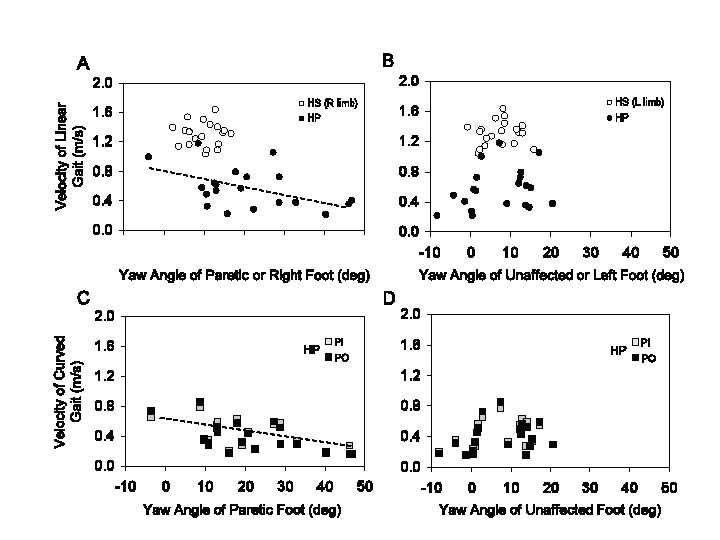
Foot yaw angle at foot fall. During linear walking, the mean yaw angles of the unaffected foot were similar to HS (unpaired t-test, p = 0.83), but there was an increase in data dispersion in HP (Levene’s test, p < 0.001) (Fig. 3A). On the other hand, the yaw angle was larger (more extra-rotated) in the paretic than non-paretic foot (unpaired t-test, p < 0.001) (compare Fig. 3A and B). No significant relation was observed between yaw angle of the unaffected foot and strength. However, yaw angle of the paretic foot was negatively correlated with strength (y = –0.45x + 48.3, R2 = 0.29, p < 0.05) (Fig. 4A).
Fig. 3. Frequency histograms of foot yaw angles at mid-stance in healthy subjects (HS) and hemiparetic stroke patients (HP). (A) Right limb for HS and unaffected limb for HP; (B) left limb for HS and paretic limb for HP. The sound side of HP has been arbitrarily compared with the right lower limb of HS and the affected side of HP with the left lower limb of HS (note that the yaw angles of right and left foot overlap in HS). When panels (A) and (B) are compared, it appears that the yaw angles of the paretic feet (B, black bars) span a larger range than the healthy feet (A, grey bars).
Fig. 4. (A) Relationship between foot yaw angle of the paretic limb and muscle strength of the same limb measured by the Motricity Index. (B) Relationship between the asymmetry index of the duration of the single support and foot yaw angle of the paretic limb. In (A) and (B), the regression lines have been fitted across the hemiparetic stroke patients (HP) data-points (black circles). Open circles in (B) refer to healthy subjects (HS), and show no relationship between foot yaw angle and duration of single support.
Asymmetry index. The asymmetry index of single support duration during linear walking was less than 1% in HS (no asymmetry), but had a mean value of approximately 21% in HP (unpaired t-test, p < 0.001). The asymmetry index was strongly related to the degree of yaw angle of the paretic foot (y = 0.59x + 8.65, p < 0.001, R2 = 0.50) (Fig. 4B). The walking velocity was inversely related to the asymmetry index, under both linear and circular trajectories (linear: y = –0.01x + 0.81, p < 0.001, R2 = 0.21; PI: y = –0.01x + 0.69, p < 0.01, R2 = 0.47; PO: y = –0.01x + 0.75, p < 0.001, R2 = 0.60) (not shown).
Yaw angle of the paretic foot and velocity of curved walking
When walking velocities were plotted as a function of the yaw angle of either foot in HS, no relationship was observed under either linear (Fig. 5A, B, open circles) or curved walking (not shown). This depended on the small range of yaw angles and walking velocity in HS. Conversely, across the patients, gait velocity decreased as a function of yaw angle of the paretic foot (for both linear and curved walking) (Fig. 5A, linear, y = –0.01x + 0.76, p < 0.05, R2 = 0.23; Fig. 5C, curved: PI, y = –0.01x + 0.56, p < 0.05, R2 = 0.29; PO: y = –0.01x + 0.58, p < 0.01, R2 = 0.36). No effect on velocity was evident for the yaw angle of the unaffected foot, either for linear (Fig 5B) or curved walking (Fig. 5D).
Fig. 5. (A) Relationship between velocity of linear walking and paretic foot yaw angle in hemiparetic stroke patients (HP) or right foot in healthy subjects (HS). (B) Relationship between velocity of linear walking and foot yaw angle of the unaffected side in HP (or left foot in HS). Same for walking along curved trajectories in HP (C, D). The regression lines in (A) and (C) have been fitted through the HS data-points.
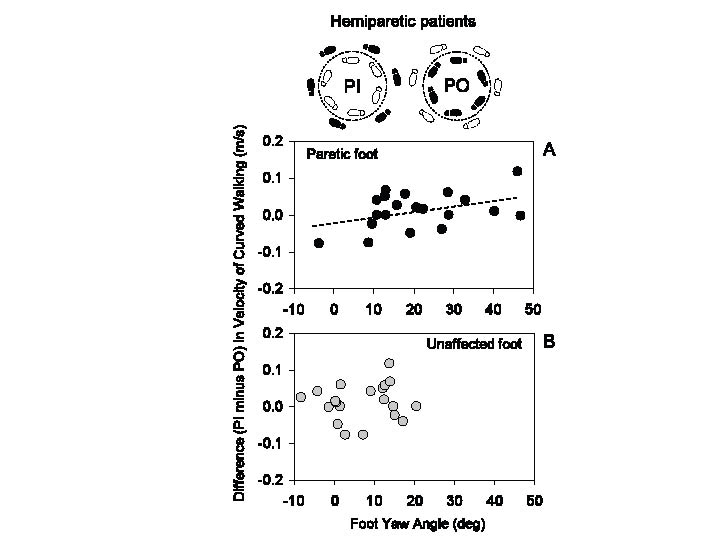
Velocity of curved walking in the patients was not different, on average, between PI or PO conditions (Fig. 5C). However, owing to the effect of the asymmetry index and Motricity Index on velocity, both broadly related to foot yaw angle at mid-stance, the result in Fig. 5C does not allow us to answer the question whether foot yaw angle per se has an effect on velocity when walking toward the paretic or toward the unaffected side. However, differences emerged when the population data variance was annulled by considering within each patient the difference in velocity (PI minus PO). A significant regression appeared (y = 0.002x – 0.02, p < 0.05, R2 = 0.19) when we plotted the individual velocity differences (PI minus PO) against the yaw angle of the paretic (Fig. 6A) but not of the unaffected foot (Fig. 6B).
Fig. 6. (A) Relationship between the difference (paretic inside (PI) minus paretic outside (PO)) in the velocity of curved walking (inset) and paretic foot yaw angle. (B) Same plot, for the unaffected foot. The slope of the regression line in (A) is significant. There is no relationship between difference in walking velocity and yaw angle of the unaffected foot.
Correlations between gait and clinical or stabilometric variables
Across the patients, there was an inverse correlation between velocity of linear walking and M-L asymmetry of CoP position during quiet stance (y = –0.009x + 0.76, p = 0.03, R2 = 0.23). There was also an inverse relation between velocity of curved walking and CoP position regardless of the direction of the circular trajectory (PI: y = –0.01x + 0.51, p = 0.09, R2 = 0.15; PO: y = –0.01x + 0.53, p = 0.03, R2 = 0.24). When the difference in velocity in either direction was plotted against the CoP position, there was a tendency for the patients, whose CoP position was shifted toward the unaffected side, to walk a little faster when they turned toward the paretic side (y = –0.001x – 0.003, p = 0.12, R2 = 0.13). No significant correlations were found between gait variables and sway area, sway path or CoP A-P position.
DISCUSSION
Walking implies the capacity to make turns or produce extended bouts of locomotion along curved or circular trajectories, from going around a table to negotiating a congested area. Whereas HS have no problem in moving around such obstacles, patients with motor impairments are more uneasy with turning than proceeding along a linear trajectory. In HP, body weight asymmetry (18, 19), impaired inter-segmental coordination (20), axial rigidity and postural instability (20), as well as impaired control of task-specific neural mechanisms (17, 22, 23) may add to the turning difficulties often present in elderly HS (15) or in other motor impairments (24). The production of curved walking for an extended period of time may pose problems as a result of patients’ medio-lateral instability and difficulty in shifting the centre of foot pressure from one foot to the other (25, 26). Furthermore, imposing curved walking, and hence asymmetries in leg and trunk movements, might increase cognitive load and emphasize problems in gait control (27). This study aimed to identify problems in gait during continuous circular walking; in particular, in view of the altered mechanical and neural contribution of the paretic limb in the control of posture (8, 18, 28), we wondered whether difficulties in walking along circular trajectories might be related to the direction of locomotion, towards or away from the paretic side.
Our patients showed a wide range of walking velocities, from as low as 0.2 m/s to almost normal velocity. However, within their walking capacities, they were able to walk along both straight and curved trajectories, without manifesting severe additional problems during curved walking. This is in keeping with the observation that chronic hemiparetic patients cope well with the challenges of varied environments (29). Furthermore, our patients walked continuously for 1 min, but showed negligible fatigue-related degradation between the first and second part of the trial (both for straight and curved walking) (30). During curved walking, the direction of rotation did not affect the average walking velocity, regardless of the inner or outer placement of the paretic foot with respect to the curved trajectory. Moreover, deliberately producing curved walking requires accurate anticipation of successive body positions: anticipation has been shown to be a problem in HP (23), but did not appear to affect the capacity to implement a circular trajectory in the present patients.
These data do not allow us to speculate on whether and to what extent the present patients had improved their walking ability over time or had learned to draw on different functional resources (22, 31) for walking along circular trajectories. We cannot exclude that medication in HP, in addition to paresis, may have affected gait velocity; however, linear walking was, in a sense, the control of curved walking for every subject and patient; moreover, there were patients walking (either curved or linear or both) with velocities superimposed on those of the slowest HS, in spite of their medications. Cadence was affected by curved walking to the same extent as in HS. This finding points to the robustness of the process of generation and appropriate modulation of the locomotor rhythm. The total distance travelled was, however, comparatively shorter along the curved than straight trajectory with respect to HS. Lower velocity and cadence require smaller displacements of the centre of mass, thereby minimizing M-L body displacements. All in all, it seems that in these chronic HP, the descending command for producing curved walking is not severely impaired or at least selectively affected: it can accommodate the emerging torques connected with the balance-threatening continuous change in direction (4, 32) and with their abnormal foot landing position (33). However, a “protective” feature is obvious, whereby the reduction of velocity during curved walking diminishes the equilibrium challenge by diminishing the centripetal force necessary for producing the circular trajectory. This diminution occurred in spite of the already low velocity of the patients, therefore in spite of an obvious floor effect. If the velocity during curved walking is plotted as a percentage of the velocity during linear walking, the percentage reduction proved to be larger in HP than HS (even if not significantly so).
Overall, the hypothesis that HP would show differences in spatial or temporal gait variables between turnings toward the paretic vs toward the unaffected side was not confirmed. If anything, a minor but non-negligible difference became obvious when the difference in curved-walking velocities within each patient (toward one side minus toward the other side) was considered. The velocity of the curved walking toward the paretic side could be slightly faster or slower than in the opposite direction depending on the yaw angle of the paretic foot: the paretic foot being extra-rotated favours turning towards the paretic side than in the opposite direction (Fig. 6A). In a sense, the extra-rotation (the most common foot attitude in HP), known to perturb linear walking (34), is less disturbing during curved walking with the paretic limb on the inside. Notably, in HS curved walking is performed by coordinated extra-rotation of the foot internal to the curved trajectory (3). In HP, this extra-rotation is the consequence of the lower limb paresis, and may have a “positive” effect when turning towards the paretic side. This might explain why little alteration in turning has previously been observed (7) when patients turn toward the paretic side.
Another non-alternative possibility is that walking velocity toward the unaffected side is reduced with respect to the opposite direction because the extra-rotated paretic foot, now external to the trajectory, is not in the most appropriate condition for propelling the body. In fact, in HS, the foot external to the trajectory is placed on the ground in an intra-rotated attitude, thereby favouring propulsion in the direction of the curved path by triceps contraction (2, 35). These considerations are supported by the observations that the single support time is much shorter for the paretic foot than for the unaffected foot and that the corresponding asymmetry index increases as a function of extra-rotation. Therefore, when the paretic foot is external to the curved trajectory, it has a less effective orientation in space, and less time to propel the body, which is further reduced as a function of the increase in extra-rotation.
Having the body weight toward the healthy side during standing did not help curved walking toward this same side (36); some of these patients walked even faster when turning to the paretic side. Postural asymmetry in HP patients is, to a large extent, adequate functional compensation for the loss of muscle strength and control of the affected side (37, 38). This postural asymmetry may also be a sensible solution for curved walking. In addition, it is possible that a trade-off occurs between shift of CoP to the unaffected side and foot extra-rotation to the paretic side, leading to no major side-related difference in turning to either direction.
In conclusion: (i) walking along curved trajectories is a demanding task in HP and should be considered as a tool for highlighting impaired gait control in HP; (ii) there is no difference in impairment in walking towards the paretic side than toward the unaffected side; (iii) the orientation of either foot at foot fall affects curved walking; (iv) the paretic foot extra-rotation and its short single support time hinder walking toward the unaffected side, whilst the same paretic foot extra-rotation favours turning toward the paretic side. Given the relative simplicity of data collection and analysis, such a circular-trajectory test and the analysis of foot orientation in the horizontal plane, in addition to the more traditional evaluation in the sagittal or frontal plane (39, 40), may be useful in the clinical setting to assess gait post-stroke adaptation and in rehabilitation.
ACKNOWLEDGEMENTS
This study was supported by Ricerca Finalizzata 2005 from the Italian Ministry of Health and by Progetti di Ricerca di Interesse Nazionale 2005 [2005059738] from the Italian Ministry of Research.
REFERENCES