OBJECTIVE: To evaluate two commonly used forms of hand training with respect to influence on dexterity and cortical reorganization.
SUBJECTS: Thirty healthy volunteers (mean age 24.2 years).
METHODS: The subjects were randomized to 25 min of shaping exercises or general activity training of the non-dominant hand. The dexterity and the cortical motor maps (number of excitable positions) of the abductor pollicis brevis muscle were evaluated pre- and post-training by the Purdue Peg Board test and transcranial magnetic stimulation, respectively.
RESULTS: After shaping exercises the dexterity increased significantly (p ≤ 0.005) for both hands, mostly so in the non-dominant hand. The cortical motor map of the abductor pollicis brevis muscle shifted forwardly into the pre-motor area without expanding. After general activity training, no significant improvements in dexterity were found for the non-dominant hand. The cortical motor map of the non-dominant abductor pollicis brevis muscle expanded significantly (p = 0.03) in the posterior (sensory) direction.
CONCLUSION: These results indicate that shaping exercises, but not general activity training, increase dexterity of the trained non-dominant hand in parallel with a shift of location of active transcranial magnetic stimulation positions. Shifts of active cortical areas might be important for the interpretation of brain plasticity in common behavioural tasks.
Key words: dexterity; hand training; healthy subjects; transcranial magnetic stimulation; plasticity; cortical shift.
J Rehabil Med 2010; 42: 789–794
Correspondence address: Christina Brogårdh, Department of Rehabilitation Medicine, Skåne University Hospital, SE-221 85 Lund, Sweden. E-mail: christina.brogardh@skane.se
Submitted October 1, 2009; accepted May 25, 2010
INTRODUCTION
Reorganization of cortical areas of the adult human brain can occur short- and long-term after injuries, exercises, immobilization and sensory stimulation. Increased use/tactile stimulation of the hand in animals (1, 2) and humans (3–5), may enlarge and/or shift representational cortical areas, whereas long-lasting immobilization (6) or deprivation of sensory feedback (7) has been shown to decrease representational cortical areas. Neural plasticity can occur quickly, and simple repeated thumb movements may induce cortical representational changes and changes in motor performance after only 5–30 min of exercising (8, 9).
In patients, rehabilitation of the upper extremity after stroke can induce cortical reorganization in parallel with an improvement in motor function (10, 11), especially after a forced-use technique called constraint induced movement therapy (CIMT) (12), in which the motor output area of the contralateral (injured) hemisphere has been shown to increase (5, 13–15), as examined by transcranial magnetic stimulation (TMS). TMS is a non-invasive method that has been used increasingly to map motor cortical organization in humans (16) and to measure reorganization, e.g. after stroke (17).
CIMT consists of intensive, repetitive training of the more affected arm/hand for several hours per day and of wearing a restraint on the less affected arm 90% of waking hours for a period of 2–3 weeks (12, 18–20). The exercises in CIMT consist of shaping, i.e. practise of adaptive tasks, whereby behavioural goals are attained in small steps with a gradual increase in difficulty and clear feedback (number of repetitions or time spent) as well as of general activity training, e.g. practise of household tasks. Taub and co-workers have emphasized that shaping exercises are of outmost importance in the CIMT concept (21) and this has been confirmed by others (22). On the other hand, some groups (13, 23) have reported that forced-use therapy (FUT) without shaping also improves upper extremity function in chronic stroke. Since the shaping exercises require a one to one relationship between patient and therapist it is important systematically to examine the efficacy of shaping vs general activity training, both on motor performance and on cortical reorganization.
The aim of the present study was to compare how a brief period of shaping exercises or of general activity training, commonly used in stroke rehabilitation, influences dexterity as well as the size and location of the cortical motor area of the abductor pollicis brevis (APB) muscle. Since it is extremely difficult to assemble a large group of patients with homogenous stroke lesions, we chose to use the function of the non-dominant hand of healthy humans as a model for hand training and for cortical plasticity, employing TMS of the contralateral cerebral hemisphere to explore possible cortical reorganization.
SUBJECTS AND METHODS
Subjects
Subjects were recruited mainly among students and employees from Umeå University, Sweden. One subject was an industrial worker. Information about the study was given in verbal and written form. Before entering the study the subjects completed a questionnaire concerning sex, age, occupation, handedness, possible impairments of the non-dominant hand, intake of medication, pregnancy and activities of fine motor practise with both hands. Criteria for inclusion were: (i) being healthy and (ii) age range 18–40 years. Exclusion criteria were: (i) epilepsy, (ii) cardiac pacemaker, (iii) pregnancy, (iv) metal objects implanted in the skull, (v) fixed tooth brace, (vi) reduced joint motion or sensory impairment of the non-dominant hand, (vii) proficient typing, (viii) regularly playing musical instruments (such as piano, violin, saxophone, etc.) or playing video games > 30 min per day (to avoid the effects of regular hand exercises outside the study) and (ix) cortical motor threshold > 80% of the maximal intensity of our TMS stimulator (see below).
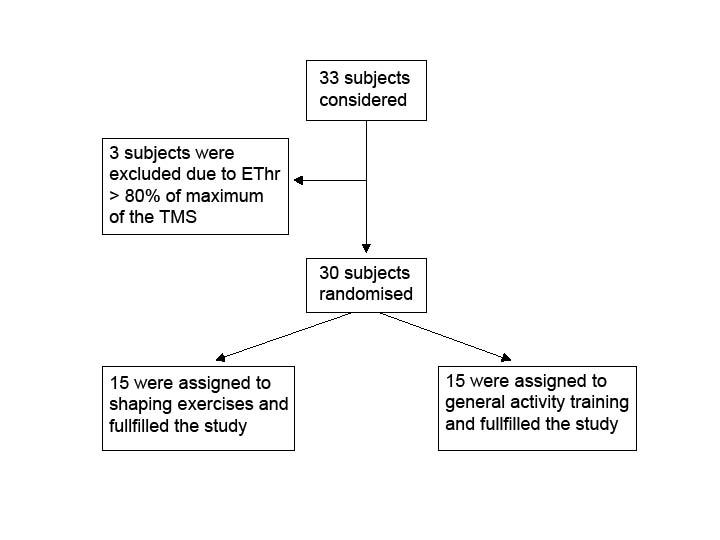
Thirty-three healthy persons were recruited and 30 volunteers (25 women, 5 men; mean age 24.2 years) fulfilled the study criteria after informed consent. Three subjects were excluded because their minimal excitability threshold for the APB muscle was higher than 80% of the maximal stimulation intensity of our equipment for magnetic brain stimulation (Fig. 1). Out of the 30 subjects, 26 were right-handed and 4 were left-handed. The number of subjects included in the study was based on a power calculation (a power of 90%, an alpha of 0.05, 14 subjects per group, with a total of 28 subjects, allowing a mean difference between groups in number of active scalp positions on TMS of 6.4; Stat-Mate®). This difference in active positions represents 60% of previously published data from training effects in patients with stroke (14).
Fig. 1. Flowchart of subjects through the study. EThr: minimal excitability threshold for the abductor pollicis brevis muscle, TMS: transcranial magnetic stimulation.
Protocol and randomization
Immediately before the hand training, the subjects underwent cortical mapping of the APB motor area of the contralateral (non-dominant) hemisphere by TMS, followed by dexterity testing (see below) by investigators (FWJ, FN), who were blinded with regard to the hand training allocation. The subjects were then randomized into two groups. The randomization procedure was prepared in advance by an independent secretary who was not otherwise participating in the study, using a random table and inserting paper sheets marked A or B in consecutively numbered, but otherwise unmarked, envelopes, sealed by the same person. When a new subject arrived for training, the trainer (CB) opened the next numbered envelope and initiated the type of training indicated (see below). After hand training, cortical mapping and dexterity testing were repeated by the independent investigators. The physiotherapist (CB) who supervised the hand training was not present during the TMS mapping and dexterity testing. The duration of a whole test and training session was 3 h. The code was broken only after analysing the raw data from the dexterity tests and the TMS cortical maps from all subjects.
Hand training
Hand training was initiated within 5 min after the cortical mapping and dexterity testing, either as shaping (group A) or as general activity training (group B). The shaping consisted of 3 standardized exercises with progressively increasing difficulty in small steps: (i) to put coins through a slot (10 coins/trial × 5 followed by 15 coins/trial × 5), (ii) to turn playing-cards over (10 cards/trial × 5 followed by 15 cards/trial × 5), and (iii) to put rubber bands around an oval can (7 rubber bands/trial × 5 followed by 10 rubber bands/trial × 5). Each shaping exercise included verbal and written feedback (time of task performance). The general activity training consisted of 3 tasks: (i) to lay and clear a table once for 4 persons (including table-mats, glasses, plates, knives, forks, spoons, serviettes and candles in approximately 7 min), (ii) to wipe the table with a damp duster (in approximately 5 min) and (iii) to draw lines between dots to form a figure on a paper (in approximately 9 min).
The subjects performed the hand training with the non-dominant hand for 25 min (including 1 min rests between the tasks) and simultaneously wore a mitt (constraint) on the dominant hand to avoid compensatory movements. The physiotherapist (CB) supervising the hand training had extensive experience of CIMT in patients with stroke.
Dexterity testing
The subjects’ dexterity was evaluated with the Purdue Peg Board test. This test has been shown to have sufficient validity and reliability (24). It includes 4 tasks; (i) with the right hand: place as many pins as possibly from a right-hand cup into the right hand row in 30 s; (ii) with the left hand: place as many pins as possibly from a left-hand cup into the left hand row in 30 s; (iii) with both hands: pick up a pin from the right hand cup with the right hand and at the same time pick up a pin from the left hand cup with the left hand and place as many pins as possible in rows in 30 s and (iv) assembly: pick up a pin with the right hand, a washer with the left hand, a collar with the right hand and another washer with the left hand. Assemble as many units as possible in 60 s.
Each task was assessed 3 times: using the right hand, the left hand, both hands and the assembly task. The data for the 3 trials for each task was pooled and the averages were used in the statistical analysis. Dexterity testing lasted approximately 15 min (before and after hand training).
Cortical mapping
The TMS technique is known to be safe and painless (25) and has been reported to be reliable and reproducible (26). Cortical mapping was performed using a focal 8-shaped coil (Medtronic MCF-B65, Medtronic A/S, Skovlunde, Denmark) connected to a Medtronic® MAGPRO X100 transcranial magnetic stimulator. Before the mapping, the questions about exclusion criteria were repeated. The subjects were asked to remove jewellery, keys, mobile phone, wallet and credit cards. They were seated comfortably in a chair with the arms resting on a pillow in the lap. A thin cap made of latex was put over the subject’s head where landmarks (nasion, inion, vertex-aural line, outlines from the ears) secured the position. A grid with 1 cm spacing was drawn on the cap over the non-dominant hemisphere where the vertical lines were numbered from the vertex-aural line and the horizontal lines alphabetically from the vertex. An ear protector was put in the outer ear on the mapping side to reduce the noise artefact from the coil.
Motor evoked potentials (MEPs) were recorded with the add-on electromyography module (MEP Monitor) of the MAGPRO X100, utilizing electromyography single-use surface electrodes placed on the non-dominant hand for the duration of the experiment; over the APB muscle (active electrode), on the distal part of the thumb (reference electrode) and on the forearm (ground). The subjects were instructed to relax the muscles during the assessments.
During cortical mapping the coil was moved systematically over the grid positions on the lateral aspect of the non-dominant hemisphere and kept in the rostro-caudal direction with the grip pointing backwards. The stimulation was performed with biphasic pulses of 280 µs width at 0.5 Hz stimulus rate. It was started with 20% of the maximal initial magnetic pulse field intensity (dB/dt:38 kiloTesla/s) and was increased progressively in steps of 5% to identify the position(s) with the minimal excitability threshold (EThr) for the APB muscle. The EThr was defined as the lowest intensity producing discernible MEPs with constant latency from the surface recordings in 5 out of 10 stimulations (27) and its location constituted the Hot Spot (HS). Thereafter, at an intensity of 20% above the motor threshold of the APB muscle, a map was obtained, delivering 5 stimuli at 0.5 Hz for the positions surrounding the HS. If at least 1 out of the 5 stimuli gave a MEP response with an appropriate latency, the position was considered positive. The MEP at each positive position was photographed for further analysis using a digital camera mounted on a tripod. The area around the HS was searched systematically in grid steps of 10 mm until negative positions were found in all directions. The mapping sessions before and after training were identical (each using about 200–300 stimuli) and lasted between 45 and 60 min.
Data analysis
The data were analysed using the Statistical Package for the Social Sciences (SPSS) version 12.0 Software for Windows (SPSS, Chicago, IL, USA). The number of responsive scalp positions (map size) of the APB muscle were analysed as well as possible shifts in direction of responsive positions after hand training. Shifts were analysed from grid positions of new as well as eliminated positions after training in relation to the HS position. The mean and standard deviations (SD) for the neurophysiological data as well as for the pooled dexterity data (Purdue Peg Board test) were calculated within and between the groups. The 1-sample t-test was used to analyse the treatment effect on dexterity within the groups, and the paired sample t-test was used to analyse the treatment effect on dexterity between the groups, respectively. A p-value < 0.05 was considered statistically significance and a 95% confidence interval (CI) was used when applicable.
Ethics
The research protocol was approved by the Medical Ethics Committee of Umeå University, Sweden (No. 05-029M).
RESULTS
Training effects on dexterity
The pooled data of the Purdue Peg Board test for the two groups before and after the hand training are presented in Table I. After the shaping exercises the participants in group A significantly increased their dexterity in all 4 tasks. The mean difference in placing pins with the non-dominant hand increased by 1.27 pins (CI 0.69–1.84; p < 0.001); with the dominant hand by 1.22 pins (CI 0.44–2.01; p = 0.005); with both hands by 1.00 pins (CI 0.58–1.42; p < 0.001) and for the assembly by 4.82 units (CI 2.89–6.76; p < 0.001).
| Table I. Mean and standard deviation (SD) of the pooled data (3 trials) of the Purdue Peg Board test before and after hand training for groups A and B, respectively |
| Number of pegs | Group A (shaping) | Group B (activity) |
| Before training | After training | Before training | After training |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| Non-dominant hand | 15.6 (1.1) | 16.8 (1.3) | 15.6 (1.8) | 15.9 (1.9) |
| Dominant hand | 16.5 (0.7) | 17.7 (1.3) | 17.0 (1.9) | 17.6 (2.2) |
| Both hands | 13.2 (1.0) | 14.2 (1.1) | 13.4 (1.3) | 13.5 (2.0) |
| Assembly | 43.5 (4.5) | 48.4 (3.6) | 43.9 (6.2) | 47.0 (6.4) |
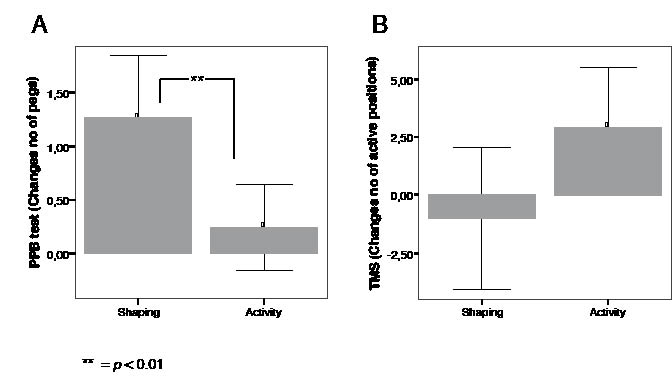
After the general activity training (group B) no statistically significant improvement in dexterity was found in the trained non-dominant hand (mean difference 0.25 pins: CI –0.16 to 0.65; p = 0.213) or in both hands (mean difference 0.11 pins: CI –0.50 to 0.72; p = 0.709), but a small but significant difference was found in the dominant hand (mean difference 0.58 pins; CI 0.02–1.13; p = 0.042). Furthermore, there was a statistically significant improvement in the assembly task (mean difference 3.09 units; CI 1.57–4.61; p < 0.001). Thus, the improvement in dexterity was particularly prominent in group A (after shaping exercises). The mean difference in placing pins for the trained non-dominant hand was also significant between the groups (p = 0.007; Fig. 2A).
Fig. 2. Bars of mean difference with 95% confidence interval before and after shaping exercises and after general activity training of: (A) dexterity (number of pegs) for the non-dominant hand, as measured by the Purdue Peg Board test (PPB test) and (B) the number of active (responsive) scalp positions upon transcranial magnetic stimulation (TMS) of the contralateral hemisphere for the abductor pollicis brevis muscle on the non-dominant side. **p < 0.01.
Size of cortical motor output area
The change in number of active (responsive) scalp positions over the non-dominant hemisphere for the APB muscle before and after hand training for the two groups can be seen in Fig. 2B. The motor output area was significantly enlarged only after the general activity training (group B). The number of active scalp positions for group B was a mean number of 18.5 (SD = 6.28) before training and 21.4 (SD 5.97) after training (mean difference 2.93 positions; CI 0.33–5.54; p = 0.03). The number of active scalp positions after shaping exercises (group A) was a mean number of 22.0 (SD 6.18) before training and 21.0 (SD 5.78) after training (mean difference –1.0 positions; CI –2.06 to +4.06; NS).
New and eliminated number of responsive scalp positions
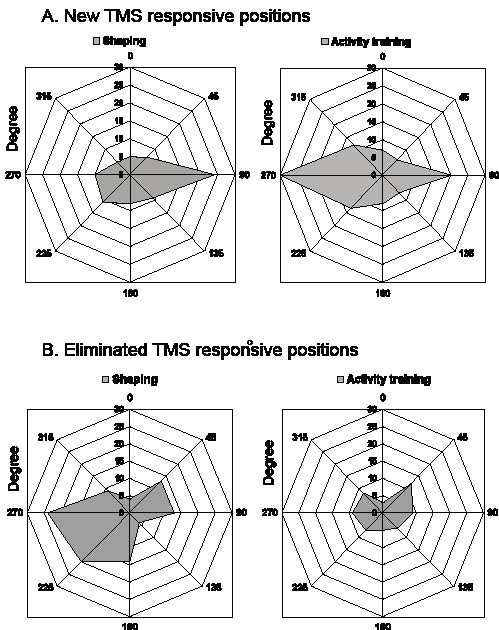
The HS was located about 30 mm anterior to the vertex-aural line and consisted of 1–2 active positions for both groups. To analyse possible shifts of active (responsive) scalp positions after the hand training we employed sectorial maps in steps of 45 degrees emerging from the HS and with the vertex (medial) direction defined as 0o and that of the nasion (anterior direction) as 90o. The new active scalp positions after shaping (group A, Fig. 3A) were found mainly in the anterior direction. In this group, the HS moved by 10 mm in the anterior direction in 7/15 subjects, downwards in 1 subject but did not change in 7 subjects (mean difference 6.7 mm: CI –0.2 to 13.5; p = 0.05). After general activity training (group B, Fig. 3A) the new active scalp positions were mainly found in the posterior direction from the HS. The HS in this group moved posteriorly and/or downwards by 10 mm in 7/15 of the subjects, anteriorly/upwards in 5 subjects and did not change in another 3 (mean difference 2.7 mm: CI –2.2 to 7.6; p = 0.262). The elimination of scalp positions (Fig. 3B) was considerable after the shaping exercises, whereas only a few, evenly distributed positions were eliminated after general activity training.
Fig. 3. Polar diagrams, illustrating (A) the number of new and (B) of eliminated abductor pollicis brevis cortical responsive positions after shaping (left-hand diagrams) and after general activity training (right-hand diagrams), compiled from all subjects and illustrated in steps of 45 degrees from the hot spot (origo). y-axis: number of positions. 0° = medial direction, 90° = anterior direction, 180° = lateral direction, 270° = posterior direction. TMS: transcranical magnetic stimulation.
During the cortical mapping 3 participants experienced discomfort, became dizzy, nauseated or developed a headache. Since there were individual landmarks on the caps, these subjects were allowed to remove the cap after the first stimulation session, if it was not possible to keep it on. This usually eased the discomfort.
DISCUSSION
The results of our study in healthy subjects indicate that less than 30 min of shaping exercises improved dexterity of the trained non-dominant hand as well as of the dominant hand and of bimanual tasks. After general activity training, we found a statistically significant improvement mainly in the bimanual task (assembly). After (but not before) training, the difference in dexterity in the non-dominant hand between subjects receiving shaping exercises (group A) and those receiving general activity training (group B) was highly significant (p = 0.007).
Both shaping exercises and general activity training, commonly used hand training modalities in rehabilitation after stroke, induced rapid cortical reorganization of the APB muscle motor area in the non-dominant hemisphere. The reorganization was not merely an increase in the number of responsive positions, as has been described previously after training in normal subjects (3–5) as well as in patients with cortical injuries from stroke (5, 13, 14). After the shaping exercises the motor cortical area of the contralateral ABP muscle shifted anteriorly into the pre-motor area in half of the subjects (Fig. 3) in parallel with improved dexterity of the trained non-dominant hand. A possible explanation of the anterior shift after shaping exercises could be that this mode of hand training involves the pre-motor area to improve motor control of the hand (i.e. the dexterity). This finding is in agreement with that of Kim et al. (28), who reported new active positions mainly in the anterior direction, i.e. in the contralateral pre-motor and motor areas in parallel with improved hand coordination after CIMT, as measured by functional Magnetic Resonance Imaging (fMRI). Shifts of cortical representation areas have also been reported after prolonged electrical sensory stimulation in normal subjects (29) and (long-term) in skilled racquet players (30). Furthermore, a study in patients with subcortical stroke (17) has found a strong positive correlation between the magnitude of a cortical map shift and the grip strength in the affected hand.
Interestingly, Morgen et al. (31) studying cortical activation patterns associated with stereotyped repetition of simple finger movements found task-specific decreases in activity, both in the contralateral motor cortex and in the inferior parietal lobule on fMRI, compatible with the location of eliminated positions after the shaping exercises in our study. Flor et al. (32), studying the effects of a sensory stimulation programme in the amputation stump for persons with phantom limb pain found that the sensory cortical representation of areas surrounding the phantom shrank to resume a more normal pattern in parallel with an improved sensory function.
Conversely, in those subjects receiving general activity training, the motor cortical area expanded posteriorly (into sensory areas) (Fig. 3A), and the HS moved posteriorly/inferiorly in half of those subjects. However, this expansion was not accompanied by improved dexterity of the trained non-dominant hand. It cannot be excluded that the cortical expansion in the posterior direction represents another dimension of motor learning than that tested by the Purdue Peg Board test (i.e. eye–arm-hand coordination). The general activity training exercises were partly based on arm movements rather than on specific hand function training, with the exception of “lay and clean a table”, which included grip and release movements.
Our subjects performed the hand training with their non-dominant hand, in order to mimic a new learning situation as much as possible. It is known that implicit knowledge (new learning) rather than explicit knowledge (deliberate recollection of information) will have an impact on the rapid plasticity of the cortical output maps to the muscles involved in a task (33). Since it has been reported that there are insignificant interhemispheric asymmetries of hand muscle representations in healthy subjects (34), we enrolled both right- and left-handed subjects. Interestingly, in both group A and B, the dexterity to perform the assembly task increased, as did the dexterity of the dominant hand (even if mainly in group A), indicating that an interhemispheric transfer and/or familiarization with the PPB test may have occurred to some extent. Another explanation could be that the dominant hand, which is normally used in dexterity tasks, adapts more rapidly in the dexterity test.
The shaping exercises in our study were selected from a battery of shaping exercises for patients with stroke with moderate functioning of the paretic arm. Some of the exercises were modified to a more difficult level since we studied healthy subjects. It would perhaps have been desirable to perform the hand training sessions for longer time periods than utilized here. However, since the whole test procedure lasted approximately 3 h for each subject, it was not possible for practical reasons to train the subjects for more than 30 min. Longer follow-up would also have been relevant, but was not possible for practical reasons.
Even if significant changes in cortical shift after shaping exercises were found, it might be argued that the cortical shift was within the magnitude of normal variation (35). However, our study indicates that the cortical reorganization differs depending on the mode of hand training performed, even if a limitation might be that the changes observed are based on hot-spot location rather than on centre of gravity calculations. The direction of the cortical shift, rather than the size of the cortical area, might thus be an important parameter in evaluating brain plasticity and seems to be related to the improved dexterity observed. In this context, it is interesting to note that studies of sensory discrimination in humans have found a positive correlation between the degree of cortical shift of neuroelectric sources and an improved somatosensory function (36, 37). The possibility that a few of the subjects removed their caps between the TMS sessions would introduce an uncertainty with respect to the positions of the motor areas, is negligible, because this occurred rarely in both groups of the 30 subjects studied and therefore would not cause a systematic bias.
We chose to investigate healthy subjects since it would be very difficult to enrol a large homogenous group of patients with stroke with the same location, size and type of brain injury necessary for the TMS component of the study. It might be argued that the shaping training influenced the functions assessed by the PPB test more than did the general activity training. It should be remembered, however, that the 2 types of training used were selected because they are common in clinical hand training practise, not because they activate the APB muscle to a similar extent. Our results cannot automatically be translated to patients with cortical or subcortical injuries e.g. stroke. It would therefore be interesting to evaluate the effects of shaping exercises vs general activity training on dexterity in a group of patients with stroke. However, it is reasonable from our behavioural and motorcortical data to assume that shaping exercises, as apart from general activity training, are effective to improve dexterity, confirming previous anecdotal evidence from Taub et al. (21).
In conclusion, this study shows that dexterity of hand movements seems to increase after a short period of shaping exercises rather than after general activity training in healthy adults. The activity training expands the number of active TMS positions of the APB muscle into more posterior (sensory) parts of the contralateral sensorimotor cortex, whereas the shaping exercises shift the ABP motorcortical area into pre-motor areas. Hence, change of location, not merely the size of motor areas, may be an important factor in evaluating cortical plasticity in humans.
ACKNOWLEDGEMENTS
The authors would like to thank all the subjects who participated in this study. We also thank Lars Bergmark, MD, PhD, Department of Clinical Neurophysiology, Umeå University, for technical advice and Peter Sojka, MD, PhD, Department of Community Medicine and Rehabilitation, Umeå University for medical support. The study was supported by the Medical Faculty, Umeå University.
REFERENCES