OBJECTIVES: A prospective study of 91 consecutive traumatic brain injury admissions to rehabilitation over a 2-year period to determine factors impacting on rehabilitation charges.
METHODS: Discharge records of 91 adult traumatic brain injury patients comprising total unsubsidized billings for each completed inpatient rehabilitation episode were used to derive total charges. Co-variates analysed included demographic, acute traumatic brain injury and rehabilitation variables including the Modified Barthel Index score.
RESULTS: The total median rehabilitation charge per episode was S$7845.50 (range: S$970.55–$44,817.20) [1 Euro = S$2.10]. The top 3 contributory median total charges/episode included bed, board and nursing (S$5616.00), occupational therapy (S$606.00), and physical therapy (S$526.00). Patients with lower admission Glasgow Coma Scale scores, longer post-traumatic amnesia duration, dysphagia and medical complications during rehabilitation, lower admission Modified Barthel Index scores, longer acute and rehabilitation length of stay had significantly higher rehabilitation charges (p < 0.001). Using multiple regression analyses, only rehabilitation length of stay and change in Modified Barthel Index were significantly correlated with total rehabilitation charges (p < 0.001).
Discussion: Measures to reduce rehabilitation length of stay, to prevent medical complications, to facilitate transfers to rehabilitation, and expedient discharge planning may help to reduce rehabilitation charges.
CONCLUSION: This study has potential implications for healthcare resource planning for traumatic brain injury rehabilitation.
Key words: head injuries; traumatic brain injury rehabilitation; charges; billing; length of stay.
J Rehabil Med 2010; 42: 27–34
Correspondence address: Karen Sui-Geok Chua, TTSH Rehabilitation Centre, 17 Ang Mo Kio Ave 9, Singapore 569766, Republic of Singapore. E-mail: Karen_Chua@ttsh.com.sg
Submitted February 16, 2009; accepted September 23, 2009
INTRODUCTION
Traumatic brain injury (TBI), according to the World Health Organization, will surpass many diseases as a major health problem and leading cause of disability by the year 2020. With an estimated 10 million people affected annually worldwide by TBI, the burden of mortality and morbidity of TBI makes it a major public health problem, and both young and old segments of the population are affected (1). In addition, it has also been estimated that ~43% of TBI survivors had developed long-term disabilities (2). Even mild TBI gives rise to chronic neurobehavioural and psychosocial sequelae, which may not be appreciated immediately after onset of TBI (3).
A recent survey of 10 nations in the Europe Union revealed that the costs of TBI ranked second in most countries after hip and lower limb fractures (4). In the USA, the charges of treating and rehabilitating TBI are recognized to be high, and represent a significant portion of the nation’s annual medical care expenditure. Kreutzer et al. (3) had demonstrated yearly increases in TBI charges at a rate of 7% per annum from 1990 to 1996, and this was offset by corresponding decreases in rehabilitation length of stay (LOS) of 8% (3.65 days) annually (3, 5). Due to physical and reimbursement pressures on a stretched healthcare system from the ageing population, with its resultant increases in elderly TBI, increasing complexity of neurorehabilitation and the explosion of novel treatments and technologies, reductions in hospitalization LOS are often accompanied by increases in charge (3, 5). In the current climate, of infinite demand for healthcare, and limited resources amidst global economic recession, such a situation is clearly unsustainable and potentially jeopardizes the appropriate delivery of TBI rehabilitation.
TBI research in the past 30 years has demonstrated sustainable functional and social improvements, such as return-to-work, return-to-home or societal roles and personal autonomy using a variety of acute treatment approaches and rehabilitation (3, 6).
In addition, following evidence-based guidelines, such as the Brain Trauma Foundation guidelines for the treatment of moderate to severe head injuries, has been shown to significantly reduce mortality and charge savings related to hospitalization. Estimated annual savings were in the region of US$262 million for medical charges, US$43 million for rehabilitation charges and lifetime societal charges of US$3.84 billion and estimated mortality reduction of 3607 lives annually (7). A new climate is thus emergent, where it is prudent not only to study the evidence for medical treatments, but also to consider the costs of medical treatments within the context of potential risks and benefits. There has also been recent interest in cost-effectiveness of TBI management as well as TBI rehabilitation among developed countries (3, 8, 9).
The situation in South-East Asia is critical due to the absence of health economic publications on TBI charges and its predictors and the need to study the impact TBI has on healthcare charges. Unlike Western healthcare systems, there is a much lower per capita spending on healthcare and less structured or developed national healthcare insurance and compensation policies (10). Yet, TBI remains the commonest cause of disability in those under the age of 35 years of age locally (11).
Hence, the objectives of this study were to:
• Determine the total and distributed charges during acute inpatient rehabilitation in a single tertiary TBI rehabilitation unit.
• Determine if there were demographic, clinical, rehabilitation or functional variables or predictors, which correlated with total rehabilitation charges.
• Compare these variables with available published data.
MATERIAL AND METHODS
Study design
This was a prospective case series study involving 91 hospitalization discharge case records of consecutive TBI admissions to a single inpatient brain injury rehabilitation facility from 1 February 2002 to 30 June 2004. Prior to data collection and analyses, this study obtained ethics approval from institutional review boards. The study did not receive any external funding.
Patients
The inclusion criteria included: consecutive admissions of those patients with completed discharge records; aged > 17 years; with first-ever acute TBI; admitted directly from acute referring hospitals. The diagnosis of TBI was made by the admitting neurosurgeons or emergency room physicians and confirmed by neuroimaging (computer tomography (CT) or magnetic resonance imaging (MRI) brain scans) within 3 hours of acute admission.
The exclusion criteria were: patients who had incomplete financial data; those admitted from the community for functional reconditioning or treatment of secondary medical complications; those who had interruptions to their rehabilitation stay by transfers off the unit due to medical or neurosurgical instability; and those who did not complete rehabilitation for any reason.
Rehabilitation programme
In Singapore, patients with acute head injury are typically admitted to the inpatient TBI rehabilitation unit from various acute neurosurgical units in the country. The facility is situated within a stepped-down care unit located 12 km from the main hospital. The inpatient rehabilitation programme consisted of 3 h of therapy per day, 5.5 days per week of physiatrist-led multidisciplinary co-ordinated rehabilitation based on the neurodevelopmental (NDT) approach. Standard rehabilitation interventions included rehabilitation nursing care, mobility and gait training, activities of daily living (ADL) training, caregiver training, pain management, comprehensive spasticity interventions, post-traumatic amnesia (PTA) charting and management of agitation and other behavioural disorders related to TBI, reality orientation therapy, psychosocial counselling, prescription of orthotics and adaptive aids as well as social work interventions related to discharge planning. Weekly staff conferences from admission until discharge were conducted for all patients.
Outcome measures
Total rehabilitation charges derived from unsubsidized total hospital billings were used as the main outcome measure, as these were the most robust means of obtaining the total rehabilitation charges per episode of hospitalization. The final figures were extracted on rehabilitation discharge and did not represent actual amounts billed to healthcare providers or patients. Unsubsidized charges were used to remove the effects of national healthcare subsidies, ranging from 0% to 80%, given to the large majority of inpatients at the study centre, which is a public hospital.
Total charges were further divided into component charges, including:
• Bed, board and nursing charges, which included the room and board charges in a typical rehabilitation ward. These also included professional physician charges that were not charged separately.
• Rehabilitation therapy charges were subdivided into charges derived from billings from physiotherapy, occupational therapy, and combined speech and psychological therapies.
• Specialized rehabilitation investigations, including videofluoroscopies, fibre-optic endoscopic examinations for swallowing disorders, spasticity-related injections and off-site neuroimaging examinations (CT or MRI brain scans).
• Radiological charges performed on-site during rehabilitation, which included plain X-rays, intravenous urograms, venous ultrasound Doppler studies and diagnostic ultrasound examinations.
• Laboratory charges, including routine tests for full blood counts, electrolyte panels, endocrine panels, etc.
• Standard and non-standard drug charges from intravenous, oral or other parenteral drugs during inpatient rehabilitation stay.
• Consumables, such as diapers, intravenous fluids, drip sets, intravenous cannulas, wound dressings and urinary catheters, etc.
• Surgeries performed off-site during the rehabilitation stay included placement of percutaneous gastrostomy tube, suprapubic catheterizations, peripherally inserted central catheters, etc.
• Services utilizing specialized orthotics and appliances such as lower limb orthotics, walking aids, resting limb splints, etc.
Clinical variables
These included demographic variables, such as age, gender, racial distribution (Chinese, Malay, Indian, others), date of injury, date of admission and discharge from inpatient rehabilitation. The latter were used to compute the acute LOS and rehabilitation LOS (days). Discharge disposition included discharge to the community (own home or relatives’ home) and nursing homes.
TBI injury variables included the aetiology of TBI (motor vehicle accident, fall, assault, others), and predominant type of TBI based on admission CT scans read by neuroradiologists. These were classified into diffuse axonal injury (DAI), lobar contusion, traumatic subarachnoid haemorrhage (SAH), subdural haematoma or extradural haematoma (SDH/EDH) and those with mixed lesions.
Severity of the TBI was measured by admission post-resuscitation Glasgow Coma Scale (GCS) scores from 3to 15 and reclassified into the following categories of severity: severe (GCS 3–8), moderate (GCS 9–12) and mild (GCS 13–15).
In addition, the depth of PTA was measured within 48 h of admission to inpatient rehabilitation using the Westmead Post-Traumatic Amnesia Assessment Scale (11). Daily scores were obtained by resident psychologists until the patient emerged from PTA or was discharged from rehabilitation. The total duration of PTA duration included the time from TBI till the first day the patient attained 3 consecutive full scores of 12/12 prior to discharge. If the patient was still in PTA at discharge, this duration was calculated from the time of TBI till the date of discharge as PTA was not scored beyond discharge. The severity of TBI using PTA duration was classified as mild (< 24 h), moderate (1–7 days), severe (1–4 weeks) and very severe (more than one month).
Acute clinical variables included the presence of concomitant polytrauma (spine or limb fractures, pulmonary or abdominal injuries and spinal cord injury), need for initial neurosurgical interventions or tracheostomy.
The presence of dysphagia at rehabilitation admission requiring either nasogastric tube feeding or modified diets for transitional feeding and occurrence of any medical complications during rehabilitation, such as pneumonia, urinary tract infection, sepsis, cardiovascular or venous thromboembolic events or decubitus ulcerations were documented.
As the centre was awaiting its licence and approval for the Functional Independence Measure (FIMTM) instrument, global rehabilitation outcome on admission and discharge for this study was measured using the Modified Barthel Index (MBI) (scores from 0 to 100). The MBI was charted within 72 h of admission and discharge by therapists and nurses, and the gain in MBI was calculated as the discharge – admission MBI score (12, 13).
Statistical analyses
For continuous outcomes, such as charge per discharge, unit charge and LOS in rehabilitation unit, we performed a natural logarithmic transformation, as the distribution were positively skewed, and there was strong departure of the distribution from normality. We then used the ordinary least squares regression model to examine factors that were associated with each outcome. Starting from the most significant variable identified in the univariate analysis, we added the next most significant variable, and used the likelihood ratio test to examine whether inclusion of a new covariate helped improve the fit of the model. Both univariate and multivariate analysis were performed. Data analysis was performed in Stata V9.2 (College Station, TX, USA) and all tests were conducted at the 5% level of significance.
RESULTS
Patients and acute injury variables
In total, there were 192 consecutive brain injury admissions during the study period from 1 February 2002 to 30 June 2004. Twenty-four were excluded from the analyses due to inability to fulfil diagnostic criteria due to age and non-TBI diagnoses. Furthermore, 77 cases (40.1%) were excluded from the analysis due to incomplete financial data (43), transfers off the unit during rehabilitation (26), and failure to complete rehabilitation due to at-own-risk discharges (8).
In all, 91 completed discharge records with complete financial data (68 males, 23 females, mean age 39.4 years, standard deviation (SD) 16.8 years, range 18–77 years) were available for analysis. The male:female ratio of 2.9:1 was typical of TBI cohorts in general and majority of TBI were related to motor vehicle accidents (64.8%) and falls (27.5%). The majority suffered severe injuries, with 98.9% having a PTA duration of > 24 h, although only 54.8% (49 patients) had admission GCS scores of 8 or less. The mean rehabilitation LOS was 29.9 days (SD 21.8) (Table I).
| Table I. Demographic and clinical variables of inpatients with traumatic brain injury (TBI) (n = 91) |
| Variable | Frequency n (%) |
| Gender Male Female | 68 (74.7) 23 (25.3) |
| Race Chinese Malay Indian Others | 66 (72.5) 11 (12.1) 11 (12.1) 3 (3.3) |
| Cause of TBI Motor vehicle accident Fall Assault Others | 59 (64.8) 25 (27.5) 2 (2.2) 5 (5.5) |
| Predominant type of TBI Diffuse axonal injury Lobar contusion Traumatic SAH SDH/EDH Mixed lesions | 27 (29.7) 9 (9.9) 9 (9.9) 39 (42.9) 7 (7.7) |
| Admission Glasgow Coma Scale Mild (3–8) Moderate (9–12) Severe (13–15) | 49 (53.8) 26 (28.6) 16 (17.6) |
| Discharge disposition Home Nursing homes/others | 85 (93.4) 6 (6.6) |
| Received acute neurosurgical intervention Yes No | 45 (49.5) 46 (50.5) |
| Presence of polytrauma Yes No | 63 (69.2) 28 (30.8) |
| Presence of dysphagia requiring NGTMD Yes No | 23 (25.3) 68 (74.7) |
| Presence of initial tracheostomy Yes No | 12 (13.2) 79 (86.8) |
| Presence of medical complications at rehabilitation |
| Yes No | 20 (22) 71 (78) |
| Presence of ventriculoperitoneal shunt Yes No | 1 (1.1) 90 (98.9) |
| Severity of post-traumatic amnesia duration < 1 day ≤ 1–7 days 8–31 days ≥ 1 month | 0 (0.0) 1 (1.0) 27 (29.7) 63 (69.2) |
| | Mean (SD) | [Range] |
| Age Admission Glasgow Coma Scale Duration of PTA Admission Modified Barthel Index Discharge Modified Barthel Index Acute lenght of stay Rehab lenght of stay Total lenght of stay | 39.4 (16.8) 8.3 (3.9) 62.2 (44.6) 63.3 (27.1) 82.9 (18.3) 34.9 (28.6) 29.9 (21.8) 68.3 (47.1) | [18–77] [3–15] [7–244] [0–100] [8–100] [2–186] [2–129] [15–245] |
| EDH: extradural haemorrhage; NGTMD: nasogastric tube or modified diet feeding; PTA: post-traumatic amnesia; SAH: subarachnoid haemorrhage; SD: standard deviation; SDH: subdural haemorrhage. |
All except 11 patients (12%) showed gains in MBI on discharge (95% confidence interval: –23.2 to –15.9, p < 0.001) and the median change in MBI per day was 0.55 points (inter-quartile range (IQR): 0.23–0.95). These 11 patients scored > 90 points on the MBI on admission to rehabilitation and the lack of change was likely due to the ceiling effects of MBI and insensitivity of the MBI to cognitive gains achieved during inpatient rehabilitation.
Rehabilitation charges
The total mean charges per rehabilitation episode were S$10,055.01 (SD 8031.47) [1 Euro = S$2.10]. Due to the skewness of the data, total median charges per rehabilitation episode were reported at S$7845.46 (range: S$970.55–44,817.20). The median charges per day were S$327.40 (IQR: 293.80–360.70) and the median charge per unit change in MBI S$491.10 (IQR: 305.50–797.90) (Table II).
| Table II. Breakdown of total inpatient rehabilitation charges (unsubsidised costs) |
| Variable | Median cost (S$)† | 25th PC | 75th PC |
| Total rehabilitation charges | 7,845.5 | 3,517.2 | 13,533.7 |
| Bed, board, nursing | 5,616.0 | 2,912.0 | 8,920.6 |
| Consumables | 43.8 | 14.6 | 244.3 |
| Radiology | 32.1 | 0.0 | 190.9 |
| Rehabilitation therapy | 1,395.0 | 645.0 | 2,590.4 |
| Physiotherapy | 526.0 | 267.0 | 1,208.8 |
| Occupational therapy | 606.0 | 263.0 | 994.7 |
| Speech therapy | 140.0 | 0.0 | 316.0 |
| Medications | 79.1 | 18.7 | 227.9 |
| Laboratory cost | 121.8 | 0.0 | 289.7 |
| Specialized investigations | 283.7 | 126.0 | 585.0 |
| Surgery* | 0.0 | 0.0 | 0.0 |
| Orthotics and appliances* | 0.0 | 0.0 | 0.0 |
| *Excessive zeros encountered. †1 Euro = S$2.10. PC: percentile. |
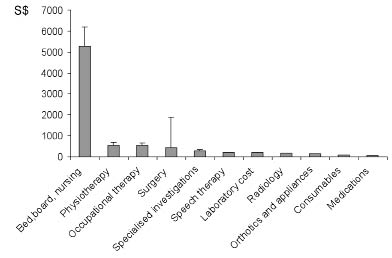
The top 10 distributed (component) charges represented by their geometric means are shown in Fig. 1. These included: bed, board and nursing charges (S$7497.94), followed by physiotherapy (S$549.34), occupational therapy (S$539.41), surgery, which was performed for 5 patients (S$438.48), specialized investigations (S$286.61), speech and language therapy (S$207.41), laboratory charges (S$200.38), radiology charges (S$160.26), orthotics and appliance charges (S$153.21) and drugs (S$67.97) (Table II).
Fig. 1. Distributed costs of traumatic brain injury rehabilitation. [1 Euro = S$2.10.] Note: Geometric means and 95% confidence interval.
Correlational analyses
Univariate analyses for factors associated with total charge per episode are shown in Table III. Patients with more severe injuries represented by lower GCS scores, longer PTA duration and longer acute LOS (p < 0.05, p < 0.001, p < 0.001, respectively) had significantly higher total rehabilitation charges, as well as those with dysphagia requiring tube feeding or modified diets on admission, medically complicated patients, and those who had tracheostomies (p < 0.001). Age, injury type using neuroimaging and the presence of neurosurgical procedures, or associated polytrauma, such as fractures, were not associated with higher total charges of rehabilitation (Table III).
| Table III. Univariate factors associated with total charges per episode |
| Factors | Median cost | Coefficient | 95% CI | p-value |
| Age | 0.06* | 0.003 | –0.01 to 0.01 | 0.488 |
| Glasgow Coma Scale | –0.22* | –0.04 | –0.08 to 0.00 | 0.043 |
| PTA duration | 0.66* | 0.01 | 0.01 to 0.01 | < 0.001 |
| Type of TBI | | | | |
| Diffuse axonal injury | 11,664 | Reference | | |
| Lobar contusion | 3,411 | –0.78 | –1.37 to –0.18 | 0.011 |
| Traumatic SAH | 7,845 | –0.48 | –1.07 to 0.11 | 0.109 |
| SDH/EDH | 8,543 | –0.26 | –0.64 to 0.13 | 0.184 |
| Mixed lesions | 6,545 | –0.38 | –1.04 to 0.27 | 0.244 |
| Neurosurgery required | | | | |
| Yes | 9,652 | Reference | | |
| No | 6,357 | –0.24 | –0.57 to 0.09 | 0.152 |
| Presence of dysphagia requiring NGTMD |
| Yes | 13,534 | Reference | | |
| No | 6,140 | –0.83 | –1.17 to –0.50 | < 0.001 |
| Presence of medical complications at rehabilitation |
| Yes | 17,467 | Reference | | |
| No | 6,182 | –1.01 | –1.35 to –0.67 | < 0.001 |
| Presence of initial tracheostomy |
| Yes | 14,906 | Reference | | |
| No | 6,575 | –0.84 | –1.30 to –0.38 | < 0.001 |
| Presence of ventriculoperitoneal shunt | | | | |
| Yes | 28,254 | Reference | | |
| No | 7,823 | –1.34 | –2.90 to 0.22 | 0.092 |
| Admission Modified Barthel Index | –0.66* | –0.02 | –0.02 to –0.01 | < 0.001 |
| Discharge Modified Barthel Index | –0.32* | –0.01 | –0.02 to 0.00 | 0.004 |
| Change in Modified Barthel Index | 0.71 | 0.03 | 0.02 to 0.04 | < 0.001 |
| Acute LOS | 0.4 | 0.01 | 0.01 to 0.02 | < 0.001 |
| Rehab LOS | 0.95 | 0.03 | 0.03 to 0.04 | < 0.001 |
| Associated injuries | | | | |
| Yes | 8,438 | Reference | | |
| No | 7,000 | –0.24 | –0.59 to 0.12 | 0.188 |
| Total charges analysed on the natural logarithmic scale due to the skewness in the distribution. *Spearman correlation presented. CI: confidence interval; EDH: extradural haemorrhage; LOS: length of stay; NGTMD: nasogastric tube or modified diet feeding; PTA: post-traumatic amnesia; SAH: subarachnoid haemorrhage; SDH: subdural haemorrhage; TBI: traumatic brain injury. |
Multivariate regression analyses showed that, for total rehabilitation charges, only rehabilitation LOS followed by a change in MBI was significantly correlated with charges (p < 0.001) (Table IV). Sub-analyses of factors affecting rehabilitation LOS showed that those with longer duration of PTA, lower admission MBI, longer acute LOS, and dysphagic patients, those with tracheostomy, medical complications and lobar contusions had significantly higher rehabilitation LOS (p < 0.001) (Table V).
| Table IV. Multivariate factors associated with total charges per episode (with and without length of stay (LOS)) |
| Factors | Coefficient | 95% CI | p-value |
| Total charges per episode | | | |
| Rehab LOS | 0.027 | 0.024 to 0.031 | < 0.001 |
| Change in MBI | 0.012 | 0.007 to 0.016 | < 0.001 |
| Total charges per episode excluding Rehab LOS | | | |
| Change in MBI | 0.020 | 0.015 to 0.026 | < 0.001 |
| PTA duration | 0.007 | 0.005 to 0.010 | < 0.001 |
| Presence of medical complications at rehabilitation | | | |
| Yes | Reference | | 0.005 |
| No | –0.392 | –0.659 to –0.124 |
| Total charges analysed on the natural logarithmic scale due to the skewness in the distribution. CI: confidence interval; MBI: Modified Barthel Index; PTA: post-traumatic amnesia. |
| Table V. Univariate factors associated with rehabilitation length of stay (LOS) |
| Factors | Median LOS | Coefficient | 95% CI | p-value |
| Age | 0.127* | 0.010 | –0.01 to 0.16 | 0.176 |
| Glasgow Coma Scale | –0.192* | –0.35 | –0.08 to –0.76 | 0.090 |
| PTA duration | 0.727* | –0.12 | –0.08 to 0.01 | < 0.001 |
| Type of TBI | | | | |
| Diffuse axonal injury | 32 | Reference | | |
| Lobar contusion | 11 | –0.70 | –1.27 to –0.12 | 0.018 |
| Traumatic SAH | 22 | –0.47 | –1.04 to 0.10 | 0.105 |
| SDH/EDH | 27 | –0.18 | –0.55 to 0.20 | 0.350 |
| Mixed lesions | 23 | –0.24 | –0.87 to 0.39 | 0.455 |
| Neurosurgery required | | | | |
| Yes | 29 | Reference | | |
| No | 18.5 | –0.27 | –0.58 to 3.05 | 0.093 |
| Presence of dysphagia requiring NGTMD | | | | |
| Yes | 39 | Reference | | |
| No | 18 | –0.80 | –1.12 to –0.48 | < 0.001 |
| Presence of medical complications at rehabilitation | | | | |
| Yes | 49.5 | Reference | | |
| No | 19 | –0.91 | –1.25 to –0.58 | < 0.001 |
| Presence of tracheostomy | | | | |
| Yes | 47.5 | Reference | | |
| No | 22 | –0.78 | –1.22 to –0.33 | < 0.001 |
| Presence of ventriculo-peritoneal shunt | | | | |
| Yes | 85 | Reference | | |
| No | 25.5 | –1.32 | –2.83 to –0.19 | 0.086 |
| Admission Modified Barthel Index | –0.679* | –0.18 | –0.23 to –0.01 | < 0.001 |
| Discharge Modified Barthel Index | –0.38* | –0.15 | –0.02 to –0.01 | < 0.001 |
| Change in Modified Barthel Index | 0.68* | 0.03 | 0.02 to 0.03 | < 0.001 |
| Acute LOS | 0.442* | 0.01 | 0.01 to –0.02 | < 0.001 |
| Associated injuries | | | | |
| Yes | 27 | Reference | | |
| No | 24 | –0.21 | –0.56 to 0.13 | 0.223 |
| Note: Total cost analysed on the natural logarithmic scale due to the skewness in the distribution *Spearman correlation presented CI: confidence interval; EDH: Extradural haemorrhage; LOS: length of stay; NGTMD: Nasogastric tube or modified diet feeding; PTA: Post Traumatic Amnesia; SAH: Subarachnoid haemorrhage; SDH: Subdural Haemorrhage; TBI: traumatic brain injury. |
DISCUSSION
This is the first local study to document total inpatient rehabilitation charges, correlates and utilization patterns after inpatient rehabilitation following severe TBI treated at a single centre. The primary outcome was total charges per hospitalization episode billed to patients prior to computation of national subsidies rather than daily charges, as the latter reflect a derived value rather than actual inpatient hospitalization charges. Due to skewness of the data, geometric means, medians and IQR were presented in the results (Table II).
Patients
The demographic characteristics and injury types and mechanisms of TBI of this mostly severely injured population were typical of other TBI cohorts locally and for general TBI populations (2, 14–17) The smaller numbers of moderate and mild severity TBI by PTA duration were explained by such patients being discharged from inpatient rehabilitation at acute neurosurgical facilities thus avoiding transfer to our unit, which treats the more severe patients with TBI.
Charges of TBI rehabilitation
Most of the published literature worldwide and in Asia relates to the costs of treating acute TBI rather then rehabilitating TBI (6, 8, 18). From our study, the total charge of TBI rehabilitation per episode was S$10,055.01 (SD 8031) and, due to cross-country differences in how healthcare and rehabilitation charges are computed, it is difficult to draw conclusions on comparisons in costs between different countries compared with similar general TBI rehabilitation data (3, 19). Similar Asian or local comparison data were unavailable at the time of writing.
The majority of distributed charges of TBI rehabilitation were derived from bed, board and nursing charges, followed by rehabilitation therapy and surgical charges. The predominant charges by physiotherapy and occupational therapy compared with the smaller contribution from speech therapy and psychological therapies reflected the major emphasis on motor neurorehabilitation and regaining of important walking and related mobility functions that are highly desired by patients and caregivers in the acute phase of rehabilitation. In addition, inpatient speech rehabilitation focused on the evaluation and management of swallowing disorders, prevention of aspiration, and safe commencement of oral feeding in those who had a nasogastric feeding tube on admission. After removal of nasogastric feeding tubes, language therapies assumed the next priority and this is often continued into the outpatient phase. Due to the common PTA-related behavioural issues, such as agitation, poor attention span, fatigue and poor short-term memory, formal aphasia assessments are usually delayed till emergence from PTA was achieved with a reduction in agitation.
The unexpected low psychological billings in this TBI cohort could be explained by 70% of patients having a greater than one month duration of PTA, with the majority remaining confused on discharge. PTA management was generally ward-based, and serial monitoring was conducted by therapy aides and supervised by clinical psychologists. These were factored into the bed, board and nursing charges rather than billed under psychological services, hence the low consumption of psychological charges. The main psychological billings were derived from neuropsychological tests, which were conducted post-discharge following emergence from PTA and were not captured in this data-set.
The low contributions of laboratory and radiology charges were related to the relative medical stability of patients located in local stepped-down care units where transfers off the unit were mandated for medically unstable patients due to the lack of high dependency or intensive care units support within the rehabilitation unit. Similarly, charges derived from medications were also low due to the judicious use of drugs in TBI and the routine use of generic or standard medications, which are heavily subsidised in local public hospitals.
Predictors of total rehabilitation charge
Using univariate analyses, it was found that the presence of dysphagia significantly impacted on overall charges, and these were probably related to a combination of intensive nursing and dysphagia therapies, which heightened cost burdens. These included high manpower and consumable charges of 2–3 hourly bolus nasogastric tube feedings, which were the standard rehabilitation feeding regime, repeated swallowing procedures, such as videofluoroscopies, 2–3 times/day speech therapy interventions and time and effort required for the preparation of modified consistency diets. The bolus feedings allowed more physiological gastric emptying patterns compared with continuous pump feedings, facilitation of sleep-wake cycles and allowed easier mobilization out of bed for rehabilitation therapies.
In our cohort, the GCS probably underestimated injury severity, as ~99% has PTA durations of > 24 h, indicative of severe TBI. Tracheostomized patients also had higher rehabilitation charges related to higher LOS. Severity of injury as represented by admission PTA and GCS scores, lower functional status and longer acute LOS significantly impacted charges, under-writing the need for high-quality acute neurotrauma care, which may eventually reduce later rehabilitation charges. Multivariate regression analyses confirmed that rehabilitation LOS was the single most important factor in moderating charges of inpatient rehabilitation, followed by PTA duration and poorer admission functional status, which inversely correlated with charges. These findings are in agreement with earlier publications (9, 19).
Contrary to the study by Cowen et al. (19), those with fractures did not have higher charges or rehabilitation LOS related to pain management or demands for X-rays or orthopaedic reviews, as these patients had lowered mobility expectations and functional goals compared with those without fractures (p = 0.1). In addition, the availability of on-site orthopaedic reviews by visiting orthopaedists reduced the charges incurred for external reviews.
Targeted interventions to appropriately reduce LOS without compromising functional gains need to be developed within TBI programme structures. Such interventions may include appropriate goal-setting commensurate for age and severity of each TBI patient, moderation of goal setting and functional expectations for elderly TBI with poor prognostic factors or vascular co-morbidities, aggressive prevention and management of medical complications and compliance with evidence-based guidelines for TBI, which are proven to reduce mortality and inpatient hospitalization charges (4). Other potential cost-effective targets include improving dysphagia interventions by using evidence-based guidelines and avoidance of multiple or ill-timed videofluoroscopies. Managing PTA and its related cognitive-behavioural spectra may help to reduce high rehabilitation LOS often associated with agitated patients (20, 21). Optimal social work and case management services also need to be in place to expedite discharge planning and processes in order to reduce social overstayers.
This study had several limitations. Firstly, the inability to use the FIMTM instrument meant that relative contributions of motor and cognitive FIMTM impacting cost could not be determined. The Barthel index has definite limitations for functional outcome measurements due to its rapid floor and ceiling effects for inpatients and poor ability to track functional changes related to cognitive and language impairments. The small sample size performed within a single centre, which lacked the expertise to treat paediatric TBI, the missing financial data, and resultant skewness of data, may impair the ability of this data to be generalized to other TBI populations. Data from ~15% of all TBI admissions during the study period who were transferred off the unit during rehabilitation were excluded from our analyses; hence it is suspected that actual charges could be underestimated.
In addition, this data-set reflected hospitalization charges related to a predominantly severe TBI population, hence there is poor generalizability of this data to mild or moderate TBI populations. Furthermore, charges related to emergency department data are not included in this data-set, hence minor and mild TBI related charges are largely under-represented. It is known that even mild TBI is associated with chronic neurobehavioral symptoms; lost opportunities for return to work or independent living and continuing to consume healthcare resources. Outpatient billings in the community may continue years after the initial injury and these were not captured in the current study. Indirect charges related to informal social support and opportunity charges related to carer provision during inpatient and outpatient rehabilitation were not within the scope of this study. The number of episodes of healthcare delivery (e.g. therapy sessions, physician consultations and number of radiological or laboratory investigations) was also not captured.
Nevertheless, this preliminary snapshot of inpatient total charges has potential implications for rehabilitation resource planning. The results of this study could be used to derive predictive models for post-rehabilitation outcomes and rehabilitation charges in order to identify social or financial at-risk individuals or families entering rehabilitation, for early counselling and intervention. Multi-centre studies may give insights into charges nationwide, but study methodology may be fraught with difficulties due to differences in computation of charges among different institutions and variations in TBI rehabilitation programmes.
In conclusion, total unsubsidised charges are in the region of S$10,000 for an average 30-day stay, with the majority of charges derived from bed, board and nursing and multidisciplinary rehabilitation charges. Rehabilitation LOS and functional gains measured by changes in MBI were the main determinants of discharge charges. This was followed by injury severity using PTA duration and medical complications.
Comprehensive and wide-ranging interventions to reduce acute and rehabilitation LOS may reduce total rehabilitation charges. Future studies need to evolve in sophistication in order to attempt to measure the cost-effectiveness of each rehabilitation intervention through the entire continuum of TBI rehabilitation and to attempt to accurately quantify indirect charges in the post-discharge phase. In addition, studies to compare the rehabilitation outcomes and cost-effectiveness of community-based rehabilitation programmes with standard inpatient rehabilitation programmes for TBI need to be explored.
REFERENCES