Anna Bjerkefors, PhD1, Mark G. Carpenter, PhD2, Andrew G. Cresswell, PhD3 and Alf Thorstensson, PhD1
From the 1Department of Neuroscience, Karolinska Institutet, and the Swedish School of Sport and Health Sciences (GIH), Stockholm, Sweden, 2School of Human Kinetics, University of British Columbia, Vancouver, Canada and 3School of Human Movement Studies and Division of Physiotherapy, University of Queensland, Brisbane, Australia
OBJECTIVE: The aim of this study was to assess if, and how, upper body muscles are activated in a person with high thoracic spinal cord injury, clinically classified as complete, during maximal voluntary contractions and in response to balance perturbations.
METHODS: Data from one person with spinal cord injury (T3 level) and one able-bodied person were recorded with electromyography from 4 abdominal muscles using indwelling fine-wire electrodes and from erector spinae and 3 upper trunk muscles with surface electrodes. Balance perturbations were carried out as forward or backward support surface translations.
RESULTS: The person with spinal cord injury was able to activate all trunk muscles, even those below the injury level, both in voluntary efforts and in reaction to balance perturbations. Trunk movements were qualitatively similar in both participants, but the pattern and timing of muscle responses differed: upper trunk muscle involvement and occurrence of co-activation of ventral and dorsal muscles were more frequent in the person with spinal cord injury.
CONCLUSION: These findings prompt further investigation into trunk muscle function in paraplegics, and highlight the importance of including motor tests for trunk muscles in persons with thoracic spinal cord injury, in relation to injury classification, prognosis and rehabilitation.
Key words: abdominal muscles, electromyography, maximal voluntary contraction, paraplegia, posture.
J Rehabil Med 2009; 41: 390–392
Correspondence address: Anna Bjerkefors, Biomechanics and Motor Control Laboratory, GIH, Box 5626, SE-114 86 Stockholm, Sweden. E-mail: anna.bjerkefors@gih.se
Submitted September 5, 2008; accepted November 20, 2008
INTRODUCTION
Spinal cord injury (SCI) results in a complete or partial loss of ability to sense and move below the level of the injury. An SCI is associated with extensive functional impairments and most often compels the person to lifelong wheelchair usage. A primary goal for rehabilitation is to regain as much function and control of the upper body musculature as possible, to enable everyday life tasks to be performed from a sitting position. In this context the trunk muscles become critical, since they provide the necessary trunk stabilization. Trunk muscles are, however, not included in the assessment tool routinely used to classify motor function in persons with SCI, i.e. the International Standard of Neurological Classification of Spinal Cord Injury (1, 2). Thus, conclusions about motor connectivity to trunk muscles are precluded and classification of the neurological lesion level in persons with thoracic SCI will be based solely on sensory function. Whether it is possible to activate trunk muscles can be established objectively with electromyography (EMG). Since the geometry of the trunk muscles is complex, intra-muscular EMG electrodes are needed to distinguish activation of individual muscles. EMG data have indicated that persons with paraplegia can adopt alternative strategies for trunk control involving normally non-postural upper body muscles during sit-and-reach tasks (3, 4). In a recent study, we reported an ability to counteract balance perturbations and keep the trunk upright in persons with thoracic SCI (5), but no EMG was recorded to illustrate how this was actually achieved.
The main purpose of this case study was to investigate if, and how, muscles of the upper body, including deep abdominal muscles, were activated in a person with a high thoracic SCI, clinically classified as complete, (i) during maximal voluntary contractions, and (ii) in reaction to unexpected balance perturbations.
METHODS
Two healthy habitually active men participated, one with a thoracic SCI, and one able-bodied. Age, body height and mass for the person with SCI were: 29 years, 1.85 m and 73 kg, respectively. He was traumatically injured 3 years previously, with a neurological lesion at T3 level. The injury was clinically classified as complete (“A” according to the American Spinal Injury Association (ASIA) Impairment Scale) (2), i.e. no sensory or motor function in the S2–S4 segments. According to the ASIA classification (2), his motor score was 50, and his sensory scores for light touch and pinprick 44 and 37, respectively (maximal motor (100) and sensory (112) scores represent unimpaired function). A zone of partial preservation was detected in 2 dermatomes on the left side: T4 with normal sensation and T5 with impaired sensation. Age, body height and mass of the able-bodied person were: 38 years, 1.91 m, and 91 kg, respectively. Approval was granted by the Regional Ethics Review Board.
Maximal voluntary static contractions of 3–5 sec against external resistance were performed in upright sitting in the wheelchair, and included attempted trunk extension, trunk flexion, and trunk flexion with leftward and rightward rotation, as well as a Valsalva manoeuvre. Balance perturbations were performed as support-surface translations with the participants sitting in wheelchairs, which were firmly fixed to a belt that was displaced 0.60 m with an initial acceleration (peak 1.4 m/s2) followed by a 2-sec period of constant velocity and then a deceleration (5). The perturbations were triggered using a digital pulse from a CED micro 1401 and Spike 2 data collection software (Cambridge Electronic Design, Cambridge, UK) and the onset of platform acceleration measured with an accelerometer (5). Five translations in the forward (FWD) and 5 in the backward (BWD) direction were presented randomly and with varying inter-trial intervals.
EMG was recorded unilaterally (right-side) using intra-muscular and surface electrodes. Bipolar fine-wire electrodes (0.075 mm) were constructed from 7-strand Teflon-coated silver wire with 2 mm sensitive tips (Leico Industries Inc., New York, NY, USA). The tip was bent into a hook and each wire was inserted via a hypodermic needle. The precise placement was controlled with online sonographic imaging (EnVisor, Philips Electronics, Eindhoven, The Netherlands). Two wires were placed in parallel, inter-electrode separation about 5 mm, into rectus abdominis (RA), obliquus externus abdominis (OE), obliquus internus abdominis (OI), and transversus abdominis (TrA) (6). Pairs of Ag/AgCl surface electrodes (2.5 mm diameter, VIASYS Healthcare Inc. SensorMedics, CA, USA) were attached with 2 cm inter-electrode separation over the most prominent parts of pectoralis major (PM), ascending trapezius (TZ), latissimus dorsi (LD) and erector spinae (ES) at T12 level. EMG signals were amplified 1000 times (Myosystem 2000, Noraxon, Scottsdale, AZ 85254, USA), bandpass filtered 20–300 Hz and 20–500 Hz, respectively (NeuroLog 125, Digitimer Ltd, Hertfordshire, UK) and sampled continuously at 2 kHz. EMG processing was performed off-line using customized scripts developed within commercially available software (Spike2, Cambridge Electronic Design, Cambridge, UK). Using the rectified EMG signal, EMG onsets were identified as the instant at which the EMG signal exceeded one standard deviation (SD) of the baseline mean (7). All EMG onsets were visually confirmed and adjusted manually in 24% of the trials. Qualitative data comparisons were made between the 2 subjects.
RESULTS
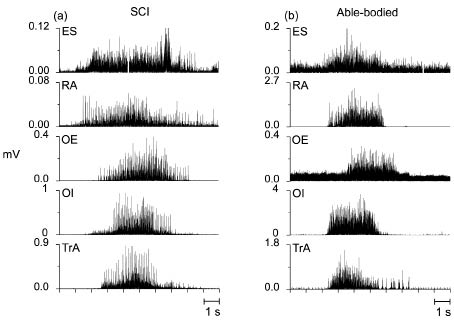
During the maximal voluntary contractions, the person with SCI was able to activate all measured trunk muscles, as shown in Fig. 1. Generally, EMG traces from the 2 participants appeared similar, but with more frequent occurrence of large individual spikes in the recordings from the person with SCI, which is probably related to a lower degree of activation in this subject (Fig. 1).
Fig. 1. Rectified electromyography traces from erector spinae (ES), rectus abdominis (RA), obliquus externus (OE), obliquus internus (OI) and transversus abdominis (TrA) recorded during single maximal voluntary isometric contractions performed by (a) the person with spinal cord injury (SCI) and (b) the able-bodied person. Note that the amplitude scales differ.
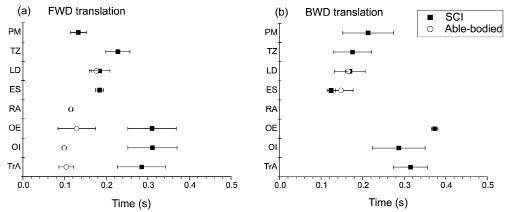
During balance perturbations, both participants were able to regain their upright upper body position in all trials. The level of activation was generally lower in the person with SCI judging from the absolute EMG values. The person with SCI appeared to use a less specific coordination pattern, i.e. with more frequent involvement of the upper trunk muscles, PM, TZ, and LD and more abundant co-activation of ventral and dorsal muscles (Fig. 2).
Fig. 2. Means and standard deviations for onset times of electromyography responses for the eight muscles investigated during (a) forward (FWD) and (b) backward (BWD) translations of the support surface in the person with spinal cord injury (SCI) (filled squares) and the able-bodied person (open circles). Zero time denotes the onset of platform translation. PM: pectoralis major; TZ: trapezius; LD: latissimus dorsi; ES: erector spinae; RA: rectus abdominis; OE: obliquus externus abdominis; OI: obliquus internus abdominis; TrA; transversus abdominis.
DISCUSSION
The main findings of this study were, firstly, that the person with a clinically diagnosed complete SCI at the T3 level could still activate his trunk muscles below this level, both voluntarily and in reaction to balance perturbations, and, secondly, that his motor responses to the balance perturbations were different from those of the able-bodied person, generally involving not only lower, but also normally non-postural upper trunk muscles.
Activation of trunk muscles with motor nerves exiting from the spinal cord below the injury level could have different underlying mechanisms. One is that the SCI was incomplete and that remaining connections might have been reinforced during rehabilitation. Studies (8, 9) have demonstrated residual sub-clinical motor function in patients with thoracic SCI clinically classified as complete. In those experiments they mainly used neurophysiological means by which they could demonstrate brain influence on spinal reflexes in the legs. Another possibility could be that activation of lower trunk muscles is induced indirectly by activation of other muscles, e.g. the upper trunk muscles. Contractions of upper trunk muscles, with intact innervation also after thoracic SCI, might lead to stretching and a subsequent reflex activation of the lower ones. Stretching of abdominal muscles could also be caused by a bulging of the abdomen induced by a contraction of the diaphragm. There might be other triggers than stretch, e.g. activation may be directed from heteronymous or even antagonistic muscle actions via neural connections adopted over time to compensate for the impairment caused by the SCI. Evidently, even though lower trunk muscles could be activated in the person with SCI, their contribution to counteracting the movement induced by the balance perturbation was insufficient and had to be complemented by recruitment of additional, normally non-postural muscles. Similar observations have been reported from experiments with sit-and-reach tasks in persons with thoracic SCI (3, 4).
As to clinical implications, the present findings point to a need to revise the current classification system for thoracic SCI to include motor tests also for trunk muscles. The development of new instruments for injury classification should occur with close interaction between basic neurophysiological and clinical research to make it valid and reliable on the one hand and user friendly on the other. In this context electromyographic recordings provide useful information and a means of validating manual tests for motor function. Finally, the new instruments should be sensitive enough to discern level of injury as well as progress with rehabilitation and training.
Conflict of interest
The authors have no disclosures.
ACKNOWLEDGEMENTS
The authors are grateful to the Swedish Inheritance Fund and the Swedish Center of Sport Research for financial support, and to Rekryteringsgruppen for Active Rehabilitation for continuously supporting the project. Special thanks are due to Rickard Levi for sharing his clinical expertise, to Tor Ansved, Håkan Strand and Craig Tokuno for skilful assistance during the experiments, and to the participants.
REFERENCES