OBJECTIVE: The aims of this study were to investigate the effects of low-frequency repetitive transcranial magnetic stimulation on patients with visual spatial neglect and to explore the potential mechanisms of visual spatial neglect.
METHODS: A total of 14 patients with prior stroke and visual spatial neglect were divided into a control group and a treatment group. The treatment group was exposed to low-frequency, repetitive transcranial magnetic stimulation for 2 weeks, twice a day, for 15 min per session. Stimuli were delivered at 0.5 Hz to the left posterior parietal cortex (i.e. position P3 according to 10–20 electroencephalogram co-ordinate systems). All patients performed a battery of tasks, including line bisection and line cancellation tests, 2 weeks before treatment, at the beginning, at the end, and 2 weeks after treatment.
RESULTS: Following low-frequency repetitive transcranial magnetic stimulation, the performance of the patients in the treatment group improved significantly. The behaviour assessment data changed with time; at time-points 2 and 3 the comparison test showed a significant difference in line cancellation and line bisection results (p = 0.003 and p = 0.027, respectively).
CONCLUSION: This study indicates that low-frequency repetitive transcranial magnetic stimulation of the unimpaired hemisphere might improve visual spatial neglect after stroke and points to the need for further studies. The results support the theory of inter-hemispheric competition in the attentional network.
Key words: visual spatial neglect, transcranial magnetic stimulation, rehabilitation.
J Rehabil Med 2009; 41: 162–165
Correspondence address: Weiqun Song, Department of Rehabilitation, Xuanwu Hospital, Capital Medical University, Beijing100053, China. E-mail: songwq66@163.com
Submitted December 2, 2007; accepted September 29, 2008
INTRODUCTION
Visual spatial neglect (VSN) is the failure to report, respond, or orient to novel or meaningful stimuli presented to the side opposite a brain lesion, when this failure cannot be attributed to either elemental sensory or motor defects (1). Due to the VSN, patients suffer serious difficulties in rehabilitation of cognitive function, motor function and prognosis (2). Unilateral VSN is common following stroke or trauma to the brain (1). Clinical anatomy shows that parietal lobe injury is common in patients with VSN (3). More precisely, the key part of the cortex related to VSN is the posterior parietal cortex (PPC) (3–5). When patients with spatial neglect are asked to perform a variety of tasks in space, they neglect the hemifield contralateral to the lesion (6). A theory of attention hypothesis (7, 8) proposes that spatial attention is linked with the inter-hemispheric competition in the attentional network. This theory proposes that a lesion in the right hemisphere induces the disorder of attention on contralateral space and impairs the restraint to the left hemisphere.
Studies suggest that the dysfunction underlying VSN might involve relative hyperactivity of the unaffected hemisphere due to release from reciprocal inhibition by its twin (9). It has been demonstrated recently that low-frequency repetitive transcranial magnetic stimulation (rTMS) over the parietal cortex of the unaffected side transiently reduces the magnitude of neglect (10). However, in that study, this positive effect was limited to the duration of rTMS train. It might therefore be worth exploring whether different magnetic stimulation parameters could induce a long-lasting improvement in contra-lesional neglect.
We applied 0.5 Hz magnetic stimulation over the unaffected side in 7 patients with neglect after stroke during a 2-week treatment period. Pen-and-paper tests were used to measure neglect before and after treatment.
MATERIAL AND METHODS
Subjects
Inclusion criteria were: those patients with right brain haemorrhage or cerebral infarction confirmed by computerized tomography (CT) or magnetic resonance imaging (MRI) and with VSN according to line cancellation and line bisection tests.
Exclusion criteria were: recurrent stroke, epilepsy, serious heart disease, serious physical disease, in vivo metal implants, such as cardiac pacemakers, increased intracranial pressure, obvious aphasia and understanding obstacles, use of tricyclic antidepressants or tranquilizers, pregnancy, age below 18 years.
A total of 14 patients, selected from January 2006 to April 2007, at the Department of Rehabilitation Medicine, Xuanwu hospital, Capital Medical University, met the study criteria. All patients gave their informed consent to participate in the study. Patients were divided randomly into a treatment and a control group.
The treatment group comprised 4 patients with cerebral haemorrhage and 3 with cerebral infarction (2 men, mean age 56.14 (standard deviation (SD) 8.99) years), mean time since stroke 38.43 (SD 15.20) days. All patients in the treatment group were right-handed except for one man.
The control group comprised 4 patients with haemorrhage and 3 with infarction (all right-handed) (6 men, mean age 64.43 (SD 12.57) years), mean time since stroke 31.57 (SD 11.47) days.
In each group there was one patient with hemianopia. The other patients had normal or corrected normal vision. The patients’ head movements were not restricted during testing. There was no significant difference between the 2 groups of patients with regard to age or duration of disease. Patient characteristics are listed in Table I.
| Table I. Patient characteristics |
| Group and patient number | Age, years /gender | Time since stroke, days | Lesion | Hemianopia, yes/no |
| Treatment group |
| 1 | 43/M | 21 | Cerebral haemorrhage in right basal ganglia | No |
| 2 | 50/M | 30 | Right basal ganglia haemorrhage Right basal ganglia and right frontal infarction | No |
| 3 | 57/F | 60 | Right thalamic haemorrhage | No |
| 4 | 59/F | 27 | Multi-infarction in right frontal, temporal, parietal, occipital cortex. | Yes |
| 5 | 70/F | 33 | Right cerebral infarction | No |
| 6 | 51/F | 40 | Right cerebral multi-infarction | No |
| 7 | 63/F | 58 | Right basal ganglia haemorrhage | No |
| Control group |
| 8 | 72/F | 24 | Right basal ganglia and temporal-parietal junction area infarction | No |
| 9 | 49/M | 29 | Right cerebral haemorrhage | No |
| 10 | 57/M | 27 | Right temporal-parietal cortex haemorrhage Right thalamus infarction | No |
| 11 | 80/M | 36 | Right basal ganglia haemorrhage | No |
| 12 | 73/M | 40 | Right basal ganglia haemorrhage | No |
| 13 | 71/M | 50 | Right basic ganglia, right corona radiata, and right parietal-occipital cortex infarction | Yes |
| 14 | 49/M | 15 | Right cerebral infarction | No |
| M: male; F:female. |
Evaluation
The study duration was 6 weeks. All participants performed line bisection and cancellation tests every 2 weeks, providing a total of 4 time-points of evaluation. Evaluation at clinical testing was blinded.
The least significant difference (LSD) method was used to evaluate the performance of the treatment and control groups in the line cancellation and line bisection tests at the 4 time-points, respectively.
Line bisection. Lines of various lengths were presented on A4 white paper. The lines were 16, 14, 12, 10 and 8 cm long. Subjects were required to find the 2 ends of the lines and then mark the mid-point. The distance between the marked point and the mid-point of the line was termed R. For deviation to the right of the mid-point, R was given a positive value; and for deviation to the left of the mid-point, R was given a negative value. The formula to calculate the VSN is R / (L/2), where L is the length of the line. The formula 20R / L was used to transfer the original value to the 10-point diagram (11).
Line cancellation. Thirty black lines (length 15–20 mm, thickness 1 mm) drawn in different directions were presented on an A4 sheet of white paper. The left and the right side each contained 15 lines. The subject were asked to mark all of the lines on the paper.
VSN was assessed according to the formula: 10 × [(30–R–L)/30] × [(R–L)/(R+L)] (R = the number of lines marked on the right side, L = the number of lines marked on the left side). In this formula, (30–R–L)/30 denoted the omission index, and (R–L)/(R+L) denoted the laterality index. The degree of neglect was calculated by multiplying the omission index by the laterality index, and then the product was normalized to the 10-point scale by multiplying by 10 for ease of comparison. So the final formula used to assess the VSN in the test was 10 × [(30–R–L)/30] × [(R–L)/(R+L)]. If the result was a positive value, it suggested neglect to the left, and a negative value suggested neglect to the right. In addition, if the subject scribbled on the right side of the paper without marking any line, the result would be 10. Conversely, if the scribbles occurred on the left side, the result would be –10 (11).
Methods
All patients were given conventional rehabilitation treatment. The treatment group also received rTMS treatment.
Measurement of motor threshold. Rapid magnetic stimulation was applied using a Magstim Rapid Transcranial Magnetic Stimulator with 2.0 T maximum field strength (Magstim Company, Dyfed, UK, P/N: 3013-00), peak intensity 2 T, time course of 1 pulse 250 µs and a figure-of-8 coil (diameter 7 cm). Motor evoked potentials (MEP) were recorded from the pollicis brevis muscle of the unaffected hand. With the muscle under resting conditions, the coil was placed on the primary motor area in the left cerebral hemisphere. The position of the coil was fine-tuned to identify where the largest MEP with the shortest latency could be achieved by use of the lowest stimulus intensity. The stimulus intensity was increased gradually until approximately 5 out of 10 consecutive stimuli elicited an MEP of approximately 50 µV. This intensity was defined as the motor threshold.
Transcranial magnetic stimulation treatment protocol. Repetitive, low-frequency stimuli were delivered with the patient lying on his or her back and the coil oriented with the handle pointing upwards. The stimulus intensity was set to 90% of the individual motor threshold and the frequency was set to 0.5 Hz. The site of stimulation was the contralateral posterior parietal cortex corresponding to P3 with regard to electro- encephalogram (EEG) 10–20. Each treatment session was 15 min long and treatments were performed twice a day for 2 consecutive weeks.
Guidance for using TMS. Safety and guidance suggestions for using TMS, as set out by Eric M. Wassermann in 1996, were followed (12). The normal index of life was recorded during the stimulation. No patient in the treatment group had any obvious adverse effects related to TMS during the study.
Statistics
Statistical software SPSS13.0 (SPSS Inc.) was used to process the data. The behaviour assessment (line cancellation test and line bisection test) data from the treatment and control groups at 4 time-points (2 weeks before TMS (time 1), beginning of TMS (time 2), end of TMS (time 3), and 2 weeks after TMS (time 4)) were processed by multivariate analysis of variance (MANOVA). Greenhouse-Geisser was used to correct the p-value if the Mauchly’s test of sphericity was significant. A repeated measurements analysis of variance was used to calculate the differences in the line cancellation test and the line bisection test between the 2 groups of patients at the 4 time-points.
Two independent samples t-test (the data was tested to satisfy the prerequisite for inspection) was used to compare the 2 groups’ behaviour data before and after treatment, respectively.
RESULTS
Change in cancellation performance of 2 groups of patients at 4 time-points
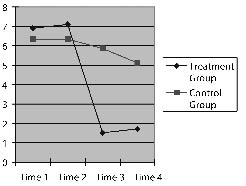
The time factor was F (1, 16) = 14.695, p = 0.001 and the interaction of time factor and group factor: F (1, 16) = 7.909, p = 0.008; both p < 0.05, which means that the measurement results at the 4 time-points differed significantly. The interaction of time factor and group factor was statistically significant. The values showed a trend to change over time and the changes differed between the 2 groups (Fig. 1).Treatment group. There was no difference between performance at 2 weeks before the treatment and at the beginning of the treatment (p =0.662). The difference between the beginning point of treatment and the end-point of the treatment was statistically significant (p = 0.003), while there was no significant difference between the end-point of treatment and 2 weeks after the treatment (p = 0.261). This indicates that the patients’ neglect symptom did not improve before the TMS intervention, while it improved significantly after that intervention. Furthermore, patients’ performance was not significantly different between 2 weeks after the TMS treatment and the end-point of the treatment (p = 0.261).
Fig. 1. Performance in line cancellation test at 4 time-points for the two groups of patients.
Control group. There was no difference between 2 weeks before the treatment and at the beginning of the treatment, or between the beginning point of treatment and the end-point of the treatment, nor between the end-point of treatment and 2 weeks after the treatment (p = 0.997, p = 0.196, p = 0.368, respectively).
Change in line bisection performance of 2 groups of patients at 4 time-points
The time factor was F (1, 17) = 4.651, p = 0.034 and the interaction of time factor and group factor: F (1, 17) = 4.602, p = 0.035; both p < 0.05, which means the measurement results at the 4 time-points differed significantly. The interaction of time factor and group factor was statistically significant. The values showed a trend to change over time and the changes differed between the 2 groups (Fig. 2).
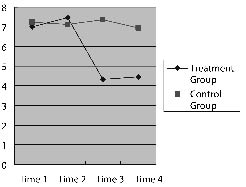
Fig. 2. Performance in the line bisection test at 4 time-points for the two groups of patients.
Treatment group. There was no difference between 2 weeks before the treatment and at the beginning point of the treatment (p = 0.545). The difference between the beginning point of treatment and the end-point of the treatment was statistically significant (p = 0.027), while there was no significant difference between the end-point of treatment and 2 weeks after the treatment (p = 0.564). This indicates that patients’ neglect symptom did not improve before the TMS intervention, while it improved significantly after the intervention. Furthermore, patients’ performance was not significantly different between 2 weeks after the TMS treatment and the end-point of the treatment (p = 0.564).
Control group. There was no difference between 2 weeks before the treatment and at the beginning of the treatment, or between the beginning point of treatment and the end-point of the treatment, or between the end-point of treatment and 2 weeks after the treatment (p = 0.600, p = 0.283, p = 0.268, respectively).
Comparison of 2 groups’ behaviour data before the treatment
The 2 groups of patients showed no difference in the results for the line cancellation test (treatment group: 6.90 (SD 3.34), control group: 6.34 (SD 3.08), p = 0.652), or the line bisection test (treatment group: 7.46 (SD 1.86), control group: 7.13 (SD 2.91), p = 0.799) before treatment.
Comparison of 2 groups’ behaviour data after the treatment
The 2 groups’ behaviour data after treatment showed that there was a significant difference on cancellation (treatment group: 1.51 (SD 2.05), control group: 5.86 (SD 2.89), p = 0.007), while in the line bisection, there was no significant difference between the 2 groups (treatment group: 4.32 (SD 2.47), control group: 7.36 (SD 3.10), p = 0.065).
DISCUSSION
Non-invasive rTMS can stimulate the cortex by inducing a painless current, thereby changing the physiological state of the brain cortex (13). rTMS has been applied as a non-invasive electrophysiological technique in treatment studies for movement disorders, epilepsy, depression, anxiety disorders, stuttering and schizophrenia (14). Existing studies have confirmed that high frequency rTMS can increase cerebral cortex excitability (4), while low-frequency rTMS can reduce cerebral cortex excitability (10).
The present study used low-frequency rTMS to stimulate the unaffected posterior parietal cortex of patients with VSN. The results show that the treatment group’s performance in both cancellation and line bisection tests did not change significantly between time 1 and time 2, which means that the patients’ VSN did not recover. While between time 2 and time 3 the comparison test showed a significant difference, which means that the TMS treatment improved patients’ VSN significantly. The comparison test between time 3 and time 4 did not show any significant difference, thus the effect of TMS on VSN was stable by 2 weeks. In the control group, the paired comparison test among 4 time-points showed no significant difference, the VSN did not recover significantly under the normal rehabilitative methods.
Moreover, 2 independent sample t-test with both groups’ behaviour data for time 1 and time 2 showed no significant difference, while the test for time 3 and time 4 showed a significant difference in line cancellation, but not in line bisection results. This may be due to the lower number of subjects in the sample. In spite of that, we can still see that the treatment group’s behaviour test scores were much better than control group’s after TMS.
The above discussion suggests that low-frequency TMS of the posterior parietal cortex can significantly improve the symptoms of VSN, with better results than conventional rehabilitation alone. No patient in the treatment group had any obvious adverse effects related to TMS during the study. In summary, our results support the theory of inter-hemispheric competition in the attentional network.
The time between the stroke and the intervention was not very long and the study sample was not very large. There is no control group using a sham stimulus to rule out the placebo effect. Thus, even if there was a difference between groups, the study does not allow general conclusions to be drawn. However, the results suggest that low-frequency rTMS might represent a complementary rehabilitative treatment in VSN.
ACKNOWLEDGEMENTS
Supported by National Natural Science Fund (30540058, 30770714), Beijing Natural Science Fund (7052030), the Organization Department of the Beijing Municipal Committee talents Fund, Beijing Science Plan project (Z0005187040191-1).
REFERENCES
1. Wang X, Chen X, Song W. The progress in the treatment of unilateral neglect with transcranial magnetic stimulation. Chin J Rehab Med 2005; 09: 715–718.
2. Cherney LR, Halper AS, Kwasnica CM, Harvey RL, Zhang M. Recovery of functional status after right hemisphere stroke: relationship with unilateral neglect. Arch Phys Med Rehabil 2001; 82: 322–328.
3. Halligan PW, Fink GR, Marshall JC, Vallar G. Spatial cognition: evidence from visual neglect. Trends Cogn Sci 2003; 7: 125–133.
4. Kim YH, Min SJ, Ko MH, Park JW, Jang SH, Lee PK. Facilitating visuospatial attention for the contralateral hemifield by repetitive TMS on the posterior parietal cortex. Neurosci Lett 2005; 382: 280–285.
5. Dambeck N, Sparing R, Meister IG, Wienemann M, Weidemann J, Topper R, et al. Interhemispheric imbalance during visuospatial attention investigated by unilateral and bilateral TMS over human parietal cortices. Brain Res 2006; 1072: 194–199.
6. Danckert J, Ferber S. Revisiting unilateral neglect. Neuropsychologia 5006; 44: 987–1006.
7. Stewart LM, Battelli L, Walsh V, Cowey A. Motion perception and perceptual learning: a magnetic stimulation study. J Electroencephalogr Clin Neurophysiol 2001; 51: 334.
8. Hilgetag CC, Kotter R, Theoret H, Claben J, Wolters A, Leone AP. Bilateral competitive processing of visual spatial attention in the human brain. Neurocomputing 2003; 23: 1.
9. Oliveri M, Rossini PM, Traversa R, Cicinelli P, Filippi MM, Pasqualetti P, et al. Left frontal transcranial magnetic stimulation reduces contralesional extinction in patients with unilateral right brain damage. Brain 1999; 122: 1731–1739.
10. Boroojerdi B, Prager A, Muellbacher W, Cohen LG. Reduction of human visual cortex excitability using 1 Hz transcranial magnetic stimulation. Neurology 2000; 54: 1529–1531.
11. Lee BH, Kang SJ, Park JM, Son Y, Lee KH, Adair JC, et al. The Character-line Bisection Task a new test for hemispatial neglect. Neuropsychologia 2004; 42: 1715–1724.
12. Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol 1998; 108: 1–16.
13. Chen ACN, Zhang W-T, Han J-S. Transcranial magnetic stimulation (TMS): physiology, psychology, brain mapping and clinical applications. Prog Physiol Sci 2004; 35: 102–106.
14. Walsh V, Cowey A. Transcranial magnetic stimulation and cognitive neuroscience. Neuroscience 2000; 1: 73–79.