OBJECTIVE: To determine the risk factors of long-term survival after stroke.
DESIGN: A prospective, hospital-based cohort study.
SUBJECTS: A total of 449 consecutive patients after acute stroke from 2 medical centres, within a 1-year period, were included.
METHODS: Dysphagia was confirmed with the water-swallow test within the first week after stroke. Data on co-morbidities and clinical risk factors were collected through chart review. Survival curves and independent risk factors were evaluated with Kaplan-Meier analysis and multivariate Cox proportion hazards analysis, respectively.
RESULTS: A total of 424 patients were followed for 10 years, and the survival was 54.2%. In univariate analysis, history of diabetes mellitus and recurrent stroke, dysphagia, urinary incontinence, cognitive impairment, tube feeding, dysarthria, and drooling were associated with higher mortality. In multivariate analysis, old age, history of recurrent stroke, and diabetes mellitus were independent predictors of long-term survival. The leading causes of death were cerebrovascular diseases and malignancy during the 10-year post-stroke period.
CONCLUSION: Dysphagia was not an independent determinant of post-stroke survival. History of recurrent stroke and diabetes mellitus were independent predictors of long-term survival. These results suggest that differential treatment strategies should be used in the different stages of stroke.
Key words: dysphagia, stroke, survival, risk factor, cause of death.
J Rehabil Med 2008; 40: 844–849
Correspondence address: Tyng-Guey Wang, Department of Physical Medicine & Rehabilitation, National Taiwan University Hospital, 7 Chung-Shan South Road, Taipei 100, Taiwan, ROC. E-mail: tgw@ntu.edu.tw
Submitted October 15, 2007; accepted July 2, 2008
INTRODUCTION
Stroke causes great mortality and morbidity among people all over the world, and Taiwan is no exception. Taiwan is an island with a population of 22.7 million, 98% of whom are Chinese. Cerebrovascular disease (CVD) is the second leading cause of death and was responsible for 13,139 deaths in the country in 2005 (1). The standardized mortality rate for stroke in Taiwan has declined over the past decades, and now averages 48.9 per 100,000 people, a figure comparable to that of many developed countries (2, 3). The direct medical expenses of this disease cost the National Health Insurance in Taiwan 370 million US dollars in 1994. There have been many studies on the risk factors for stroke in Caucasians; however, relatively few studies have focused on Chinese patients. After stroke, Chinese patients, like other Asian populations, generally have a higher rate of cerebral haemorrhage than Caucasian and black populations (2–6).
Dysphagia is one of the treatable outcomes for patients after stroke and is common among patients after acute stroke, with a prevalence ranging from 19% (7) to 81% (8), depending on the evaluation method and the amount of time elapsed between stroke and evaluation (9). By increasing the risk of chest infection (10), malnutrition, persistent disability, prolonged hospital stay, institutionalization on discharge, and 3- and 6-month mortality (7, 11), dysphagia is a marker of poor prognosis in patients after acute stroke. However, the effects of dysphagia on long-term survival are still disputed. One study reported that dysphagic patients with an absent pharyngeal swallow had a shorter survival after 6 years of follow-up (12). Shintani & Shiigai (13) identified age and dysphagia as factors determining survival in patients receiving home care. Yet another study could not correlate videofluoroscopy-confirmed aspiration with increased mortality at 30 months after stroke (14). Using multinomial logistic regression, Smithard et al. (15) found that dysphagia was associated with higher fatality only in the first 3 months, but not in the following 5 years. By studying long-term survival and related risk factors in patients after stroke with dysphagia, the aim of this study was to gain information that will enable the better allocation of resources in the future care of these patients.
Therefore, the aims of this study were: (i) to identify the 10-year survival rate and related variables predicting survival after stroke (with an emphasis on those that were dysphagia-related); and (ii) to determine the major causes of death 10 years after stroke.
MATERIAL AND METHODS
Study population
A cohort of patients after acute stroke was assembled by identifying 456 consecutive patients admitted to the Department of Internal Medicine and the Department of Neurology in 2 medical centres in Taipei,
Taiwan, over a 1-year period between January and December 1994. Both hospitals were not only tertiary referring hospitals but were also open to all citizens without referral from a general practitioner. Stroke was diagnosed by neurologists based on clinical criteria, namely the rapid development of signs of a focal disturbance of cerebral function lasting over 24 h, without an apparent non-vascular cause (16). Lesion location was classified as unilateral supratentorial, bilateral supratentorial, infratentorial, or recurrent. Stroke type, ischaemic or haemorrhagic, was also recorded from neuroimaging reports, including computer tomography (CT) or magnetic resonance imaging (MRI). Exclusion criteria were: (i) subarachnoid haemorrhage (SAH), due to a different aetiological mechanism; (ii) age younger than 18 years; (iii) failure to obtain consent; (iv) inability to receive a swallowing evaluation before discharge, for reasons including conscious disturbance and severe cognitive impairment; and (v) admission for neurosurgical intervention.
Assessment of risk factors and clinical features
Clinical variables and a detailed history of co-morbidities and known risk factors for stroke (Table I) were collected from each patient or the next of kin, in addition to medical records. The history of co-morbidities included hypertension, diabetes mellitus (DM), previous stroke or transient ischaemic attack, coronary artery disease (CAD) and hypercholesterolaemia. A history of pneumonia during the admission period was collected after the patient was discharged. The diagnosis of pneumonia was made by the attending physician based on the following variables: fever (> 38ºC), productive cough with purulent sputum, abnormal respiration, new infiltrate on chest X-ray, and positive Gram stain/culture. Dysphagia was evaluated by the water-swallow test, a bed-side examination with high sensitivity and specificity (17). Dysphagia was defined as the inability to swallow a 3–5 ml spoonful of water, experiencing coughing or choking on more than one occasion out of 3 attempts or a wet voice after swallowing (8).
| Table I. Basic demographic and clinical data of patients after stroke |
| Variables | Non-dysphagic (n = 294) | Dysphagic (n = 130) | p-value |
| Age, years (standard deviation) | 63.1 (12.6) | 68.7 (11.8) | < 0.001 |
| Male sex, % | 58 | 56 | ns |
| Duration between onset and evaluation, days (median, interquartile range) | 4.0, 4.0 | 5.0, 5.0 | |
| Co-morbidities, % |
| Hypertension | 61 | 68 | ns |
| Diabetes mellitus | 29 | 33 | ns |
| Recurrent stroke | 23 | 41 | < 0.001 |
| Pneumonia | 1.1 | 16 | < 0.001 |
| Type of stroke*, % |
| Infarct | 49 | 46 | ns |
| Haemorrhage | 30 | 35 | ns |
| Lesion of stroke, % | | | |
| Unilateral supratentorial | 76 | 55 | < 0.001 |
| Bilateral | 3.7 | 11 | 0.047 |
| Infratentorial | 4.4 | 7.7 | ns |
| Recurrent | 16 | 26 | 0.006 |
| Clinical findings, % |
| Profound limb weakness | 35 | 26 | ns |
| Sensory impairment | 33 | 59 | < 0.001 |
| Urinary incontinence | 5.6 | 33 | < 0.001 |
| Cognitive impairment | 12 | 20 | ns |
| Tube feeding | 78 | 12 | < 0.001 |
| Dysarthria | 14 | 38 | < 0.001 |
| Aphasia | 3.9 | 34 | < 0.001 |
| Impaired tongue protrusion | 15 | 35 | < 0.001 |
| Drooling | 5.3 | 22 | 0.001 |
| Impaired facial sensation | 8.6 | 15 | ns |
| Facial palsy | 34 | 73 | < 0.001 |
| Impaired cough ability | 23 | 35 | ns |
| Survival proportion, % |
| 90 days | 98 | 90 | 0.003 |
| 1 year | 96 | 83 | < 0.001 |
| 3 years | 83 | 73 | 0.023 |
| 10 years | 58 | 45 | 0.018 |
| *The summation is less than 100% due to indeterminate cases. Interquartile range is defined as the 3rd quartile minus the 1st quartile. ns: not significant. |
The definitions of other clinical parameters are as follows. Profound limb weakness: the Brunnstrom stage of the affected limbs was I–III (18). Sensory impairment: change of perception level to pin-prick on the affected side of body. Urinary incontinence: involuntary loss of urine reported by patient or caregiver (19). Cognitive impairment: score of the Mini-Mental Status Exam (20). Tube feeding: nutrition given through nasogastric tube or gastrostomy. Dysarthria: defects in articulation. Aphasia: presence of difficulties in verbal expression, auditory comprehension, repetition, or naming (7). Impaired tongue protrusion was noted if the tongue could not be protruded out of mouth or could be protruded out of mouth without full range of motion. Drooling: water spilled out of the mouth during or after water-swallow test. Impaired facial sensation: sensation of pin-prick to affected side of the face was different from the other side. Facial palsy: asymmetry during facial expression. Impaired cough ability: inadequate expulsion force during coughing (21).
Assessment of end-point
The primary end-point of this study was the death (from any cause) occurring before 31 December 2004. The method of death data collection was described previously (14). Briefly, the death of each patient was traced using the unique Citizen’s ID Number and confirmed using gender and birth date. This information was obtained from the National Death Register, Taiwan, and from death records in medical charts at the hospital. The underlying causes of death were determined according to the National Death Register and were categorized by the International Classification of Diseases-9 (ICD9) code as CVD (430–438), malignancy (140–239), heart disease (390–429,440–459), infection (001–139, 480–487), DM (250), gastrointestinal system diseases (530–579) and other diseases.
Statistical analysis
The Mantel-Haenszel χ2 test was used to make univariate categorical comparisons of the clinical features between dysphagic and non-dysphagic patients. Survival curves for all patients and for the various groups of interest were plotted using the Kaplan-Meier method. The log-rank test was used to compare rates estimated among survival curves. The association between clinical correlates and survival time after stroke was analysed with a Cox proportional hazards model. First, a univariate analysis of each possible clinical predictors of survival was performed. Then the variables with statistical significance level of 0.15 or less were retained in the subsequent multiple Cox regression analysis. In addition , age and sex were always included for adjustment. Those prognostic variables, which are taken as intermediate factors between dysphagia and survival, were eliminated in the multiple Cox regression model. If the variable required by the model was missing, the subject was eliminated from the analysis. The level of significance was set to p < 0.05 for all comparisons. Analyses were carried out using SPSS 10.0® (Statistical Product and Service Solutions Inc., Chicago, USA).
RESULTS
Among the 456 patients, 449 were included in the study at the end of 1994, and 31.6% of them were dysphagic. Twenty-five patients (5.6% of the initial cohort) lost contact during the 10-year follow-up period due to incorrect ID number, moving away or refusal to provide a complete death record. Among the remaining 424 patients (245 males, 179 females; mean age 64.8 (standard deviation (SD) 12.6) years, ranging from 21 to 92 years old), 130 (30.7%) were dysphagic, 31% had suffered a haemorrhagic stroke, 58% were male, and one-third had profound limb weakness. The median interval between stroke and clinical assessment was 4.0 days (interquartile range = 4.0 days). Generally, the demographic and clinical characteristics showed that the dysphagic group was significantly older and had higher proportion of recurrent stroke, pneumonia, bilateral brain involvement, sensory impairment, urinary incontinence, tube feeding, dysarthria, aphasia, impaired tongue protrusion, drooling and facial palsy. Table I shows the demographic and clinical details of both groups of patients.
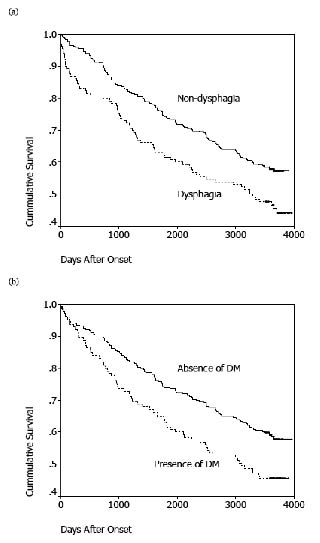
Survival proportions were 95.7%, 91.9%, 80.4% and 54.2% at 90 days, 1 year, 3 years and 10 years after stroke onset, respectively, among the 424 patients. The survival proportions between the non-dysphagic and the dysphagic groups were significantly different during the entire 10-year follow-up period (Fig. 1). In the univariate analysis, risk of death was significantly associated with a history of DM, recurrent stroke, dysphagia, urinary incontinence, cognitive impairment, tube feeding, dysarthria and drooling (Table II and Fig. 1). The effect of a given predictor on survival was expressed by the hazard ratio (95% confidence interval). A hazard ratio greater than 1 indicates a higher risk of mortality compared with the reference group. In the multivariate Cox proportional hazards model, a history of DM was the significant independent predictor of mortality after adjusting for age, sex and co-morbidities at 3 years after stroke. Age was the strongest determinant of 10-year survival. History of recurrent stroke and DM also predicted mortality after 10 years (Table III).
| Table II. Univariate Cox proportional hazards model of 10-year survival after stroke |
| Variables | Hazard ratio (95% CI) | p-value |
| Male sex | 1.00 (0.75–1.33) | ns |
| Co-morbidities |
| Hypertension | 1.21 (0.90–1.63) | ns |
| Diabetes mellitus | 1.49 (1.10–2.00) | 0.009 |
| Recurrent stroke | 1.77 (1.32–2.37) | < 0.001 |
| Pneumonia | 1.82 (0.99–3.34) | 0.056 |
| Type of stroke* |
| Haemorrhage | 1.04 (0.77–1.41) | ns |
| Lesion of stroke† |
| Recurrent | 1.62 (1.18–2.23) | 0.003 |
| Infratentorial | 0.60 (0.28–1.27) | ns |
| Bilateral supratentorial | 0.76 (0.40–1.44) | ns |
| Clinical findings |
| Dysphagia | 1.49 (1.11–2.00) | 0.007 |
| Sensory impairment | 1.05 (0.79–1.41) | ns |
| Urinary incontinence | 1.98 (1.34–2.93) | 0.001 |
| Cognitive impairment | 2.09 (1.42–3.08) | < 0.001 |
| Tube feeding | 1.53 (1.15–2.05) | 0.004 |
| Dysarthria | 1.47 (1.03–2.12) | 0.036 |
| Aphasia | 1.14 (0.71–1.84) | ns |
| Impaired tongue protrusion | 1.17 (0.96–1.41) | ns |
| Drooling | 1.68 (1.05–2.68) | 0.031 |
| Impaired facial sensation | 1.24 (0.74–2.09) | ns |
| Facial palsy | 1.10 (0.79–1.53) | ns |
| Impaired cough ability | 1.07 (0.72–1.60) | ns |
| *Infarction as reference. †Unilateral supratentorial lesion as reference. ns: not significant; CI: confidence interval. |
Fig. 1. Ten-year survival function after stroke, stratified by: (a) dysphagia and (b) history of diabetes mellitus (DM).
| Table III. Multivariate Cox proportional hazards model of survival at different time-points after stroke |
| Variables | Hazard ratio (95% CI) | p-value |
| 1 year |
| Diabetes mellitus | 2.70 (0.93–7.80) | 0.067 |
| Dysphagia | 1.90 (0.47–7.72) | 0.368 |
| 3 years |
| Diabetes mellitus | 2.13 (1.22–3.74) | 0.008 |
| Dysphagia | 1.33 (0.65–2.73) | 0.436 |
| 10 years |
| Age, per year | 1.04 (1.02–1.06) | < 0.001 |
| Diabetes mellitus | 1.68 (1.18–2.39) | 0.004 |
| Recurrent stroke | 1.51 (1.05–2.17) | 0.026 |
| Dysphagia | 1.07 (0.70–1.64) | 0.751 |
| CI: confidence interval. |
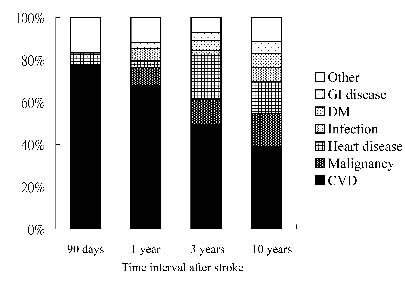
Death from CVD, including cerebral infarction, cerebral haemorrhage (CH) and SAH, was the leading cause of death in both non-dysphagic and dysphagic groups, with different time intervals from the onset of stroke. The dysphagic group had a higher percentage of deaths from DM than the non-dysphagic group (Table IV). In the whole stroke patient analysis, nearly 80% of deaths were due to CVD during the first 3 months, and the percentage dropped to approximately 40% after 10-year of follow-up. The next leading cause of death was malignancy, and it contributed 0% and 15.5% of deaths in 3 months and 10 years after stroke, respectively. Deaths from heart diseases, infection, DM, and gastrointestinal diseases increased from 6% to 31% over the 10-year period (Fig. 2).
| Table IV. Causes of death at 10 years post-stroke, stratified by the presence of dysphagia. Data are expressed as percentage (95% CI) |
| Causes of death | Non-dysphagic group | Dysphagic group | Total patients |
| Cerebrovascular disease | 38.2 (29.6–46.8) | 40.8 (29.4–52.2) | 39.1 (32.2–46.0) |
| Malignancy | 17.1 (10.4–23.8) | 12.7 (5.0–20.4) | 15.5 (10.4–20.6) |
| Heart disease | 17.1 (10.4–23.8) | 11.3 (3.9–18.7) | 14.9 (9.9–19.9) |
| Infection | 4.9 (1.1–8.7) | 9.9 (3.0–16.8) | 6.7 (3.2–10.2) |
| Diabetes mellitus | 3.2 (0.1–6.3) | 12.7 (5.0–20.4) | 6.7 (3.2–10.2) |
| Gastrointestinal disease | 5.7 (1.6–9.8) | 5.6 (0.3–10.9) | 5.7 (2.4–9.0) |
| Others | 13.8 (7.7–19.9) | 7.0 (1.1–12.9) | 11.3 (6.8–15.8) |
| Total | 100 | 100 | 100 |
| CI: confidence interval. |
Fig. 2. Constituent ratios for causes of death at different time-points after stroke. GI: gastrointestinal; DM: diabetes mellitus; CVD: cerebrovascular disease.
DISCUSSION
This prospective, hospital-based cohort study shows that dysphagia is not an independent predictor of survival during the 10-year period following stroke. Univariate analysis revealed that dysphagia, history of DM, recurrent stroke, urinary incontinence, cognitive impairment, tube feeding, dysarthria, and drooling were factors correlated with 10-year survival after stroke; however, multivariate analysis showed that only age, history of recurrent stroke, and DM were independent predictors of long-term survival after stroke. The main causes of death after stroke were CVD, malignancy, heart diseases and infectious diseases.
Dysphagia, a common disability frequently observed in brainstem or bilateral cortical lesions (22), is thought to be the predicting factor of short-term case fatality after stroke (11, 23). Wolfe et al. (24) reported that dysphagia remained an independent predictor of poor 1-year survival after adjusting for other potential confounders. Wang et al. (25) derived 2 prognostic indices based on dysphagia and other predictors in order to predict 30-day and 1-year mortality for acute ischaemic patients after stroke. However, most of these studies used the National Institutes of Health Stroke Scale (NIHSS) as a surrogate for stroke severity rather than individual symptoms. This not only simplified their prediction models, but also decreased their specificities. Some characteristics specific to lesion location cannot be differentiated in their models. Using individual symptoms as independent factors, Smithard et al. (15) followed a population-based cohort for over 5 years and concluded that the effect of dysphagia did not determine survival from 1 to 5 years after stroke onset. Dysphagia might be adjusted by individual symptoms, which is why it is not a determinant of post-stroke survival. Our results, without using the NIHSS as a predicting factor, confirmed that dysphagia was related to a poor prognosis for both short- and long-term survival (Fig. 1). After adjusting for other confounding factors, however, dysphagia was not an independent predictor of post-stroke survival. Age, recurrent stroke, and history of DM were all independent predicting factors for long-term survival after stroke (Fig. 1). This result may help clinicians to take different approaches to patients at different post-stroke stages in order to improve survival.
The water-swallow test is a commonly employed bedside evaluation for dysphagia. It has high specificity (88%) and sensitivity (70%) in detecting aspiration in dysphagic patients after stroke compared with other bedside assessment (17). It is also used in screening for dysphagia and in facilities where a videofluorographic swallowing study is not feasible (8, 22, 26).
Multivariate analysis is necessary in studying predictive factors, since colinearity and interaction between variables is quite common. If the models for analysis did not include those variables known to have important predictive effects, the results would not be reliable (27). On the other hand, those intermediate factors on the causal chain of survival should not be included in the multivariate analysis. It is accepted that dysphagia causes higher fatality by means of increased incidence of pneumonia and malnutrition (11). Thus, pneumonia and tube-feeding were not included in order to avoid bias in our model.
It was not surprising that advanced age became the strongest predicting factor among all variables over such a long-term follow-up. Besides age, recurrent stroke was also a long-term predictor in our study. Most stroke registries included first-time stroke only and found that history of transient ischaemic attack, comparable to recurrent stroke, was a determinant of survival (27). Thus, preventing recurrent stroke could contribute to longer survival.
DM is another well-known risk factor for poor prognosis (28). In a study of elderly patients suffering strokes in Taiwan, untreated DM and cognitive impairment were both independent predictors of survival (29), similar to our findings. One study specifically advocated that hyperglycaemia, without a clinical history of diabetes, was a prognostic factor for survival. It implied that untreated hyperglycaemia or undiagnosed DM were the true risks (25).
Hypertension was not significant in predicting post-stroke survival (27). A possible reason for this result might be that these patients were under appropriate treatment for hypertension during the follow-up period (30). The Nanjing Stroke Registry Program (NSRP) in China reported reduced stroke recurrence in patients with controlled, modifiable risk factors, including hypertension (31). Generally, in spite of limited studies on Chinese patients, the risk factor profiles of stroke in Chinese patients were similar to those in Western countries (2, 5, 27).
There was considerable variation in mortality among different ethnic and geographic populations (16, 32). The total 1- and 10-year mortalities were 8.0% and 45.8% in our study, respectively. These figures were still relatively low compared with other studies of Chinese patients. The 1-year mortality ranged from 10% to 14% (6, 29). The National Taiwan University Hospital Registry, which included patients from SAH and from the Emergency Department, identified a 30-day mortality of 10.9% (4). Only one study on long-term survival after a minor stroke in Italy reported a 10-year mortality of 32%, which was lower than our result. There are several reasons that might contribute to this finding. First, since CH accounted for a greater percentage of total deaths, mortality declined due to a reduction in the incidence of CH in Taiwan (2). In addition, the mortality in CH was reduced by nearly 50% in one of our recruiting hospitals. This phenomenon may be explained by less severe symptoms of CH, owing to the implementation of an anti-hypertension campaign in this country (3). Secondly, patients after stroke with severe symptoms may die before being included in such a hospital-based cohort. In spite of these differences, our results are still beneficial in managing hospital-admitted patients.
The leading causes of death were CVD and malignancy in both short- and long-term analyses. Deaths from CVD amounted to 73% and 41% of patients in the first year and 10 years post-stroke, respectively. In Taiwan, more than 95% of death certificates are issued by physicians in hospitals, and approximately 4% are issued by coroners. The causes of death listed on the certificates are relatively accurate (2). Our result was comparable to other studies in different countries (6, 33). Thus, strategies to prevent stroke recurrence and related morbidity are critical in reducing the death rate following stroke in both acute and chronic stages.
This hospital-based study suffered from limited generalization. Both hospitals in our study were tertiary referring hospitals, and the patients were not representative of the general stroke population. However, the results can still be applicable to settings similar to our study since we included all hospitalized patients after stroke from both the Internal Medicine and Neurology Wards in the 2 hospitals. Furthermore, hospital-based studies, with more correct diagnoses and comprehensive past histories, are more effective in evaluating risk profiles and assessing the clinical details (4). Thanks to the thorough registration system of the National Death Register, our proportion of loss follow-up (5.6%) was much less than that found in a comparable study (21%) (30).
In conclusion, dysphagia, history of DM, recurrent stroke, urinary incontinence, cognitive impairment, tube feeding, dysarthria and drooling were associated with poorer 10-year survival after stroke. In multivariate analysis, age, history of recurrent stroke, and DM were independent, long-term survival determinants. The leading causes of death were CVD and malignancy. Further studies, focusing on: (i) evaluating the effect of risk factor reduction and the optimal therapeutic range of the risk factors; (ii) finding the risk factors for important outcomes other than death (such as disability and institutionalization) for follow-up periods longer than 10 years; and (iii) elucidating the pathophysiological mechanisms underlying those risk factors, are all needed for healthcare planning and treatment implementation.
ACKNOWLEDGEMENTS
The authors thank Dr Hsiang-Wen Huang for statistical assistance and critical review of the manuscript, and Drs Tsz-Ching Hsu, Chih-Huei Lu and Hsin-Chui Chen for their help in data collection.
REFERENCES
1. Statistics of causes of death. Taipei: Department of Health,Taiwan; 2006.
2. Chang CC, Chen CJ. Secular trend of mortality from cerebral infarction and cerebral hemorrhage in Taiwan, 1974–1988. Stroke 1993; 24: 212–218.
3. Hung TP. Changes in mortality from cerebrovascular disease and clinical pattern of stroke in Taiwan. J Formos Med Assoc 1993; 92: 687–696.
4. Jeng JS, Lee TK, Chang YC, Huang ZS, Ng SK, Chen RC, et al. Subtypes and case-fatality rates of stroke: a hospital-based stroke registry in Taiwan (SCAN-IV). J Neurol Sci 1998; 156: 220–226.
5. Li SC, Schoenberg BS, Wang CC, Cheng XM, Bolis CL, Wang KJ. Cerebrovascular disease in the People’s Republic of China: epidemiologic and clinical features. Neurology 1985; 35: 1708–1713.
6. Liu X, Xu G, Wu W, Zhang R, Yin Q, Zhu W. Subtypes and one-year survival of first-ever stroke in Chinese patients: The Nanjing Stroke Registry. Cerebrovasc Dis 2006; 22: 130–136.
7. Barer DH. The natural history and functional consequences of dysphagia after hemispheric stroke. J Neurol Neurosurg Psychiatry 1989; 52: 236–241.
8. Meng NH, Wang TG, Lien IN. Dysphagia in patients with brainstem stroke: incidence and outcome. Am J Phys Med Rehabil 2000; 79: 170–175.
9. Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke 2005; 36: 2756–2763.
10. Nilsson H, Ekberg O, Olsson R, Hindfelt B. Dysphagia in stroke: a prospective study of quantitative aspects of swallowing in dysphagic patients. Dysphagia 1998; 13: 32–38.
11. Smithard DG, O’Neill PA, Park C, Morris J, Wyatt R, England R, et al. Complications and outcome after acute stroke: does dysphagia matter? Stroke 1996; 27: 1200–1204.
12. Bulow M, Olsson R, Ekberg O. Do dysphagic patients with an absent pharyngeal swallow have a shorter survival than dysphagic patients with pharyngeal swallow? Prognostic importance of a Therapeutic Videoradiographic Swallowing Study (TVSS). Acta Radiol 2005; 46: 126–131.
13. Shintani S, Shiigai T. Survival-determining factors in paitents with neurologic impairments who received home health care in Japan. J Clin Neurosci 2004; 225: 117–123.
14. Chen SY, Chie WC, Lin YN, Chang YC, Wang TG, Lien IN. Can the aspiration detected by videofluoroscopic swallowing studies predict long-term survival in stroke patients with dysphagia? Disabil Rehabil 2004; 26: 1347–1353.
15. Smithard DG, Smeeton NC, Wolfe CDA. Long-term outcome after stroke: does dysphagia matter? Age Ageing 2007; 36: 90–94.
16. Thorvaldsen P, Asplund K, Kuulasmaa K, Rajakangas A-M, Schroll M. Stroke incidence, case fatality, and mortality in the WHO MONICA project. Stroke 1995; 26: 361–367.
17. Tohara H, Saitoh E, Mays KA, Kuhlemeier K, Palmer JB. Three tests for predicting aspiration without videofluorography. Dysphagia 2003; 18: 126–134.
18. Brunnstrom S, editor. Movement therapy in hemiplegia: a neurophysiological approach. New York: Harper & Row; 1970.
19. International Continence Society. Standardization of terminology of lower urinary tract function. Scand J Urol Nephrol 1988; 114: 5–19.
20. Patel MD, Coshall C, Rudd AG, Wolfe CDA. Cognitive impairment after stroke: clinical determinants and its associations with long-term stroke outcomes. J Am Geriatr Soc 2002; 50: 700–706.
21. McCullough GH, Wertz RT, Rosenbek JC. Sensitivity and specificity of clinical/bedside examination signs for detecting aspiration in adults subsequent to stroke. J Commun Disord 2001; 34: 55–72.
22. Han DS, Chang YC, Lu CH, Wang TG. Comparison of disordered swallowing patterns in patients with recurrent cortical/subcortical stroke and first-time brainstem stroke. J Rehabil Med 2005; 37: 189–191.
23. Schmidt J, Holas M, Halvorson K, Reding M. Videofluoroscopic evidence of aspiration predicts pneumonia and death but not dehydration following stroke. Dysphagia 1994; 9: 7–11.
24. Wolfe CDA, Corbin DOC, Smeeton NC, Gay GHE, Rudd AG,
Hennis AJ, et al. Post-stroke survival for black-Caribbean populations in Barbados and South London. Stroke 2006; 37: 1991–1996.
25. Wang Y, Lim LLY, Levi C, Heller RF, Fischer J. A prognostic index for 30-day mortality after stroke. J Clinical Epidemiol 2001; 54: 766–773.
26. Wu MC, Chang YC, Wang TG, Lin LC. Evaluating swallowing dysfunction using a 100-ml water swallowing test. Dysphagia 2004; 19: 43–47.
27. Counsell C, Dennis M. Systematic review of prognostic models in patients with acute stroke. Cerebrovas Dis 2001; 12: 159–170.
28. Heuschmann PU, Kolominsky-Rabas PL, Misselwitz B, Hermanek P, Leffmann C, Janzen RWC, et al. Predictors of in-hospital mortality and attributable risks of death after ischemic stroke. Arch Intern Med 2004; 164: 1761–1768.
29. Wang SL, Pan WH, Lee MC, Cheng SP, Chang MC. Predictors of survival among elders suffering strokes in Taiwan. Stroke 2000; 31: 2354–2360.
30. Kimura K, Minematsu K, Kazui S, Yamaguchi T. Mortality and cause of death after hospital discharge in 10981 patients with ischemic stroke and transient ischemic attack. Cerebrovas Dis 2005; 19: 171–178.
31. Xu G, Liu X, Wu W, Zhang R, Yin Q. Recurrence after ischemic stroke in Chinese patients: impact of uncontrolled modifiable risk factors. Cerebrovas Dis 2007; 23: 117–120.
32. Feigin VL, Lawes CMM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol 2003; 2: 43–53.
33. Hardie K, Hankey GJ, Jamrozik K, Broadhurst RJ, Anderson C. Ten-year survival after first-ever stroke in the Perth community stroke study. Stroke 2003; 34: 1842–1846.