OBJECTIVE: To study the association between C-reactive protein levels and insulin resistance in patients with spinal cord injury.
DESIGN: Cross-sectional study.
SUBJECTS: Forty-two subjects who had sustained spinal cord injuries at least 6 months before enrolment.
METHODS: Circulating glucose, insulin and C-reactive protein levels were measured after 12 hours’ fasting. The homeostasis model insulin resistance index was used to evaluate insulin resistance. Insulin resistance and C-reactive protein levels were compared between complete/incomplete patients and between paraplegic/tetraplegic patients. The subjects were then divided into 3 groups (C-reactive protein levels < 1, 1–3, > 3 mg/l) to compare insulin resistance.
RESULTS: Eighteen (43%) subjects had C-reactive protein levels > 3 mg/l. The C-reactive protein levels and insulin resistance did not significantly differ between complete/incomplete or between paraplegic/tetraplegic subjects. However, insulin resistance in the high C-reactive protein group (> 3 mg/l) differed significantly from that of the other 2 groups, and there was a significant correlation between C-reactive protein and insulin resistance, with r = 0.7745.
CONCLUSION: Most young and middle-aged patients with chronic spinal cord injury with high C-reactive protein levels also have high insulin resistance, and their C-reactive protein levels have well correlated with insulin resistance.
Key words: spinal cord injury, C-reactive protein, insulin resistance, cardiovascular disease.
J Rehabil Med 2008; 40: 819–822
Correspondence address: Mao-Hsiung Huang, Department of Physical Medicine and Rehabilitation, Kaohsiung Medical University Hospital, NO.100 Tzyou 1st Road, Kaohsiung 807, Taiwan. E-mail: maohuang@ms24.hinet.net
Submitted January 16, 2008; accepted June 12, 2008
Introduction
As advances in medical management and rehabilitation technology increase the life-span of patients with spinal cord injury (SCI), the incidence of chronic disease in this population has also increased (1). The prevalence of metabolic syndrome, non-insulin-dependent diabetes mellitus (NIDDM) (2, 3) and coronary heart disease (CHD) (4) are reportedly higher in patients with SCI. As traumatic SCI usually occurs in young adults (16–30 years old), CHD and NIDDM occur at younger ages in patients with SCI than in the ambulatory population (5). Insulin resistance is known to be a major cause of metabolic syndrome and NIDDM. Patients with carbohydrate metabolism disorders are predisposed to macrovascular system diseases, which are associated with mortality and further disability in chronic SCI (6).
The aetiology of insulin resistance in SCI is unclear. The resistance may be related to increased adipose tissue (7) and lack of physical activity (8). Obesity is known to be more prevalent in patients with SCI than in the general population (9). Recent, laboratory and experimental evidence indicates that subclinical inflammation plays a central role in cardiovascular complications linked to obesity and insulin resistance (10–12). The inflammation marker C-reactive protein (CRP) is now known to be strongly associated with and independently predictive of CHD and NIDDM (13, 14). Testing for CRP is inexpensive and simple and has been endorsed by the Centers for Disease Control and Prevention (CDC) in the USA as well as the American Heart Association (AHA) (15). However, it is usually considered that CRP data should be used in conjunction with other well-known risk factors, such as cholesterol levels.
Respiratory and renal conditions are the most prevalent co-morbidities in the SCI population, and they remain important causes of mortality. However, recent studies suggest that cardiovascular disease (CVD) is now the leading cause of mortality in chronic SCI (16). Therefore, a reliable and accurate tool for identifying the risk of NIDDM, metabolic syndrome and CHD in patients with chronic SCI is vitally needed. However, it remains unclear whether CRP is useful for identifying the risk of developing insulin resistance, NIDDM or cardiovascular disease in patients with SCI. Therefore, the aims of this study are to assess insulin resistance and CRP in different levels and completeness of injury, to clarify the relationship between insulin resistance and CRP as well as the distribution of CRP levels in young and middle-aged SCI populations.
Methods
Subjects
Participants with SCI of more than 6 months duration caused by trauma episodes were recruited between April 2006 and September 2007 at our rehabilitation departments. Informed consent was obtained from all subjects. All subjects were assessed during their annual medical follow-up. Data for medications and medical history were gathered by questionnaires and from medical records. Exclusion criteria were: any symptomatic infection within the 4 weeks before enrolment, presence of NIDDM or cardiovascular disease, current use of anti-hyperlipidaemic, anti-inflammatory or glucose-regulating drugs.
Measurement of CRP levels
Blood samples were taken after participants had fasted for 12 h. Lipid profile, CRP, plasma glucose and insulin values were also recorded. Plasma concentrations of glucose were determined by the glucose oxidase method. The CRP concentrations were measured by Behring nephelometry using an N Latex CRP mono reagent (Behring Diagnostics, Marburg, Germany). CRP measurements were obtained only if the CRP level was < 10 mg/l, as values > 10 mg/l indicate an acute infection (17).
Assessment of insulin resistance
Plasma insulin concentrations were measured by immunoradiometric assay (insulin-RIA bead II, Abbott, Japan). Insulin resistance was evaluated by the homeostasis model assessment insulin resistance index (HOMA-IR) calculated as fasting plasma insulin (μU/ml) × fasting plasma glucose (mmol/l)/22.5 (18).
CRP risk categories
In accordance with Centers for Disease Control (CDC) and the American Heart Association (AHA) recommendations (15), the subjects were categorized into the following 3 groups for comparison of HOMA-IR: group I (low risk) with CRP level < 1 mg/l, group II (medium risk) with CRP 1–3 mg/l and group III (high risk) with CRP > 3 mg/l.
Neurological examination
Examination of the completeness and level of injury were performed by physiatrists at the beginning of the rehabilitation period. Patients were classified according to the American Spinal Injury Association (ASIA) impairment scale (19).
Statistical analysis
The statistical results were presented as number, percentage, mean and standard deviation (SD). SPSS Version 12.0 (SPSS Inc, Chicago, IL, USA) software was used for statistical analysis. Student’s t-test was used for group comparisons. Linear regression analysis was performed to determine the relationship between CRP and HOMA-IR. Multiple group comparisons were performed using analysis of variance (ANOVA) with post-hoc analysis. A p-value of < 0.05 was considered statistically significant.
Results
A total of 42 subjects (age range 23–50 years) with traumatic SCI were analysed. Table I shows the demographic characteristics of the study population. Two subjects had indwelling urinary catheters for neurogenic bladder dysfunction and 28 had intermittent urinary catheters. Twenty-seven subjects had a history of smoking and 3 were current smokers.
| Table I. Demographic characteristics of study patients. |
| Characteristics | |
| Number | 42 |
| Age, years, mean (SD) | 38.10 (8.57) |
| Male/female, n | 38/4 |
| Duration of injury, months, mean (SD) | 44.12 (30.44) |
| Completeness (ASIA), n (%) | |
| A | 22 (52.4) |
| B | 3 (7.1) |
| C | 11 (26.2) |
| D | 6 (14.3) |
| Level, n (%) | |
| Paraplegia | 22 (52.4) |
| Tetraplegia | 20 (47.6) |
| Current smoker, n | 3 |
| CRP, mg/l, mean (SD) | 2.65 (1.63) |
| HOMA-IR, mean (SD) | 1.34 (1.09) |
| LDL, mg/dl, mean (SD) | 131.4 (32.03) |
| HDL, mg/dl, mean (SD) | 33.42 (5.90) |
| Chol/HDL, mean (SD) | 5.68 (1.35) |
| ASIA: American Spinal Injury Association classification; CRP: C-reactive protein; HOMA-IR: Homeostasis model assessment insulin resistance; LDL: low denisty lipids; HDL: high density lipids; ChoL: cholesterol; SD: standard deviation. |
The subjects were classified by ASIA impairment scale and injury level as complete/incomplete and paraplegic/tetraplegic SCI patient groups. The CRP and HOMA-IR did not significantly differ between complete/incomplete or between paraplegic/tetraplegic SCI patients (Table II).
| Table II. C-reactive protein (CRP) and homeostasis model assessment insulin resistance (HOMA-IR) in complete/incomplete and paraplegic/tetraplegic patients with spinal cord injury. Classification according to American Spinal Injury Association (ASIA) criteria. |
| | Completeness | Level |
| Complete | Incomplete | Paraplegic | Tetraplegic |
| Number | 22 | 20 | 22 | 20 |
| Age, years, mean (SD) | 37.4 (8.12) | 38.8 (7.34) | 37.8 (5.21) | 38.3 (4.30) |
| Duration, months, mean (SD) | 44.0 (35.44) | 44.2 (30.77) | 48.5 (31.11) | 39.2 (34.17) |
| CRP, mg/l, mean (SD) | 2.71 (1.82) | 2.59 (1.46) | 2.52 (1.35) | 2.79 (1.90) |
| HOMA-IR, mean (SD) | 1.70 (1.06) | 1.41 (0.96) | 1.51 (0.86) | 1.62 (1.17) |
| All p-values were > 0.005. SD: standard deviation; CRP: C-reactive protein; HOMA-IR: Homeostasis model assessment insulin resistance. |
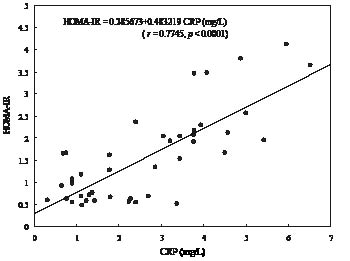
As Fig. 1 shows, CRP correlated positively with insulin resistance (HOMA-IR) in the study population (r = 0.7745; p < 0.0001). There was a progressive increase in HOMA-IR medium values with higher CRP levels in the overall population and the largest increase in the subgroup with CRP levels > 3 mg/l, as shown in Fig. 2.
Fig.1. Relationship between C-reactive protein (CRP) and homeostasis model assessment insulin resistance index (HOMA-IR) in the study population.
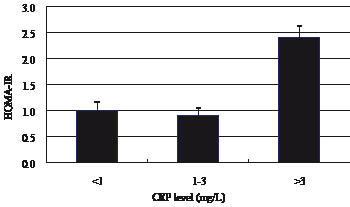
Fig. 2. Homeostasis model assessment insulin resistance index (HOMA-IR) values in spinal cord injury patient subgroups defined by C-reactive protein (CRP) risk category.
Classification according to CDC/AHA-recommended CRP cut-off points revealed 8 (19%) subjects in group I, 16 (38%) in group II, and 18 (43%) in group III, as shown in Table III. The HOMA-IR in group III was significantly (p < 0.0001) higher than that of groups I and II (Table III).
| Table III. Comparison of homeostasis model assessment insulin resistance index (HOMA-IR) in various C-reactive protein (CRP) risk categories |
| CRP level | n (%) | HOMA-IR mean (SD) | p-value* |
| I | 8 (19) | 1.00 ( 0.44 ) | < 0.0001 |
| II | 16 (38) | 0.91 ( 0.51 ) | |
| III | 18 (43) | 2.40 ( 0.93 ) | |
| Total | 42 (100) | | |
| *p-value: Group III in comparison with groups I and II. SD: standard deviation. |
Discussion
Few studies have examined the relationship between CRP values and insulin resistance in a chronic SCI population. Lee et al. (20) demonstrated elevated CRP levels in individuals with SCI (mean age 50.2 years; SD 13) who are insulin resistant and/or display components of metabolic syndrome, which suggests a clinically significant association with cardiovascular risk in this population. Moreover, some subjects in that study had taken cholesterol-lowering, anti-inflammatory or glucose-regulating drugs. To control for possible confounding factors, the present study analysed only patients with traumatic SCI and excluded subjects who had taken drugs that might have affected CRP levels (17), as well as those with history of cardiovascular disease and diabetes. Furthermore, as traumatic SCI occurs primarily in young adults, and diagnoses of CHD and diabetes occur in SCI populations at younger ages than in the general population, this study analysed a population younger than that of other studies in order to evaluate the value of early screening.
The analytical results revealed a positive correlation between CRP and insulin resistance. Inflammation may be associated with development of insulin resistance in patients with chronic SCI. Obesity is more prevalent in SCI patients than in able-bodied subjects, and even non-obese SCI subjects have a higher than average fat content (9, 21). Adipose tissue is now known to participate actively in regulating physiological and pathological processes, such as the secretion of adipokine, including cytokines (such as interleukin-6 (IL-6), tumour necrosis factor-alpha (TNF-α) and leptin), and is involved in the inflammation process (10). The CRP is an acute phase protein synthesized in the liver by hepatocytes. Production of CRP is stimulated by pro-inflammatory cytokines, particularly IL-6 and TNF-α (22). The release of adipokines by either adipocytes or adipose tissue-infiltrated macrophages causes chronic inflammation, which may play a central role in cardiovascular complications linked to obesity and insulin resistance, which are risk factor for diabetes and CHD (11). In addition, recent data indicating that CRP within atheromatous plaque (23) is a correlate of endothelial dysfunction (24) and has a direct role in cell adhesion molecular expression (25) suggest that CRP may play a direct role in the atherosclerosis process. Therefore, persistently elevated CRP may increase the risk of NIDDM or CHD in patients with chronic SCI.
The CRP levels in almost half (43%) of the subjects exceeded 3 mg/l, and these subjects were significantly more resistant to insulin than those with CRP levels lower than 3 mg/l. Bo et al. (26) documented significantly higher median insulin and HOMA-IR values in healthy subjects (aged 45–64 years) with CRP levels > 3 mg/l. Ridker et al. (27), in a cohort study of 22,000 middle-aged ambulatory males, demonstrated that those with baseline CRP levels in the highest quartile had a 2-fold and 3-fold increased risk of stroke and myocardial infarction, respectively. These effects were independent of all other lipid and non-lipid risk factors and were noted in both smokers and non-smokers. Therefore, patients with SCI with CRP levels above 3 mg/l have a higher risk for cardiovascular disease than those with lower CRP levels and should be carefully monitored. Additionally, most of the patients with chronic SCI in the examined population (mean age 38.1 years, SD 8.57) had high CRP. Therefore, early screening and management of these modifiable risk factors are recommended to reduce cardiovascular disease in this population.
Groah et al. (28) have shown that risk of cardiovascular disease increases with rostral level of SCI and severity of SCI. Neither SCI level nor SCI severity were associated with CRP or insulin resistance in our population. Rebhun et al. (29) reported that CRP became elevated but below the levels normally manifested in acute and chronic SCI, which may be related to some underlying disease state rather than to a urinary tract infection or the injury itself. In our study, CRP was found to be positively correlated with insulin resistance. However, this cross-sectional study did not determine whether the correlation between CRP and insulin resistance is temporal. Therefore, further studies of a larger population and with longer follow-up are needed in order to evaluate CRP as an indicator for indentifying patients with chronic SCI who are at risk of insulin resistance syndrome, diabetes and even cardiovascular disease.
In conclusion, most young and middle-aged patients with chronic SCI also have high CRP levels and high insulin resistance, and their CRP levels have well correlated with insulin resistance.
Acknowledgements
The authors thank the Statistical Analysis Laboratory of the Department of Clinical Research at Kaohsiung Medical University Chung-Ho Memorial Hospital for assistance with statistical analysis.
References
1. Nam CC, Odderson IR. Stroke in spinal cord injured. J Am Paraplegia Soc 1994; 17: 36–38.
2. Demirel S, Demirel G, Tukek T, Erk O, Yilmaz H. Risk factors for coronary heart disease in patients with spinal cord injury in Turkey. Spinal Cord 2001; 39: 134–138.
3. Bauman WA, Adkins RH, Spungen AM, Waters RL. The effect of residual neurological deficit on oral glucose tolerance in persons with chronic spinal cord injury. Spinal Cord 1999; 37: 765–771.
4. Myers J, Lee M, Kiratli J. Cardiovascular disease in spinal cord injury: an overview of prevalence, risk, evaluation, and management. Am J Phys Med Rehabil 2007; 86: 142–152.
5. Yakuteil M, Brooks ME, Ohry A,Yarom J, Carel R. The prevalence of hypertension, ischaemic heart disease, and diabetes in traumatic spinal cord injured patients and amputees. Paraplegia 1989; 27: 58–62.
6. Figaro MK, Kritchevsky SB, Shorr RI, Butler J, Shintani A, Penninx BW, et al. Diabetes, inflammation, and functional decline in older adults. Diabetes Care 2006; 29: 2039–2045.
7. Maki KC, Briones ER, Langbein WE, Inman-Felton A, Nemchausky B, Welch M, et al. Associations between serum lipids and indicators of adiposity in men with spinal cord injury. Paraplegia 1995; 33: 102–109.
8. Dallmeiler AJ, Hopman MT, van der Woude LH. Lipid, lipoprotein, and apolipoprotein profiles in active and sedentary men with tetraplegia. Arch Phys Med Rehabil 1997; 78: 1173–1176.
9. Weaver FM, Collins EG, Kurichi J, Miskevics S, Smith B, Rajan S, et al. Prevalence of obesity and high blood pressure in veterans with spinal cord injuries and disorders: a retrospective review. Am J Phys Med Rehabil 2007; 86: 22–29.
10. Steven ES, Jongsoon L, Allison BG. Inflammation and insulin resistance. J Clin Invest 2006; 116: 1793–1801.
11. Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006; 444: 560–567.
12. Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med 1999; 340: 115–126.
13. Pradhan AD, Manso JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001; 286: 327–334.
14. Koenig W. C-reactive protein and cardiovascular risk: has the time come for screening the general population? Clin Chem 2001; 47: 9–10.
15. Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M, et al. Centers for Disease Control and Prevention and the American Heart Association. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003; 107: 499–511.
16. Garshick E, Kelley A, Cohen SA, Garrison A, Tun CG, Gagnon D, et al. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord 2005; 43: 408–416.
17. Paul MR. C-reactive protein: a simple test to help predict risk of heart attack and stroke. Circulation 2003; 108: 81–85.
18. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Tumer RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419.
19. American Spinal Injury Association. International standards for neurological classification of spinal cord injury. Chicago: ASIA; 2002.
20. Lee MY, Myers J, Hayes A, Madan S, Froelicher VF, Perkash I, et al. C-reactive protein, metabolic syndrome, and insulin resistance in individuals with spinal cord injury. J Spinal Cord Med 2005; 28: 20–25.
21. Samsa GP, Patrick CH, Feussner JR. Long-term survival of traumatic spinal cord injury. Arch Neurol 1993; 50: 909–914.
22. Thomas B, Rifai N. High sensitivity immunoassays for C-reactive protein: promises and pitfalls. Clin Chem Lab Med 2001; 39: 1171–1176.
23. Torzewski M, Rist C, Mortensen RF, Zwaka TP, Bienek M, Waltenberger J, et al. C-reactive protein in the arterial intima: role of C-reactive protein receptor-dependent monocyte recruitment in atherogenesis. Arterioscler Thromb Vasc Biol 2000; 20: 2094–2099.
24. Fichtlscherer S, Rosenberger G, Walter DH, Breuer S, Dimmeler S, Zeiher AM. Elevated C-reactive protein levels and impaired endothelial vasoreactivity in patients with coronary artery disease. Circulation 2000; 102: 1000–1006.
25. Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation 2000; 102: 2165–2168.
26. Bo S, Gambino R, Uberti B, Mangiameli MP, Colosso G, Repetti E, et al. Does C-reactive protein identify a subclinical metabolic disease in healthy subjects? Eur J Clin Invest 2005; 35: 265–270.
27. Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997; 336: 973–979.
28. Groah SL, Weitzenkamp D, Sett P, Soni B, Savic G. The relationship between neurological level of injury and symptomatic cardiovascular disease risk in the aging spinal injured. Spinal Cord 2001; 39: 310–317.
29. Rebhun J, Madorsky JG, Glovsky MM. Proteins of the complement system and acute phase reactants in sera of patients with spinal cord injury. Ann Allergy 1991; 66: 335–338.