OBJECTIVE: To determine changes in functional independence following spinal cord injury and to evaluate the association between functional independence and physical capacity.
DESIGN: Multi-centre prospective cohort study.
SUBJECTS: Patients with spinal cord injury admitted for initial rehabilitation.
METHODS: The motor Functional Independence Measure (FIMmotor) was determined at the start of rehabilitation (n = 176), 3 months later (n = 124), at discharge (n = 160) and one year after discharge from inpatient rehabilitation (n = 133). One year after discharge, physical and social dimensions of health-related functional status (Sickness Impact Profile 68; SIP68) were determined. On each occasion, physical capacity was established by measuring arm muscle strength, peak power output and peak oxygen uptake.
RESULTS: Multi-level random coefficient analyses revealed that FIMmotor improved during inpatient rehabilitation, but stabilized thereafter. Changes in FIMmotor were associated with peak power output. Multiple regression models showed that FIMmotor and peak power output at discharge were associated with FIMmotor one year after discharge (R2 = 0.85), and that peak power output at discharge was associated with the social dimension of the SIP68 (R2 = 0.18) one year after discharge.
CONCLUSION: Functional independence improves during inpatient rehabilitation, and functional independence is positively associated with peak power output.
Key words: activities of daily living, spinal cord injury, rehabilitation, exercise test, complications.
J Rehabil Med 2008; 40: 812–818
Correspondence address: Janneke A. Haisma, Department of Rehabilitation Medicine, Erasmus MC, University Medical Centre, PO Box 2040, 3000 CA Rotterdam, The Netherlands. E-mail: j.haisma@erasmusmc.nl
Submitted September 20, 2007; accepted May 29, 2008
INTRODUCTION
Spinal cord injury (SCI) affects previously undemanding daily activities. Patients may suddenly rely on the assistance of others for seemingly straightforward tasks, and may need to adapt to the prospect of functional dependence (1). Independent functioning is a key to being active and socially involved, and may contribute to a sense of control over one’s life (2, 3). Rehabilitation professionals require insight into changes in functional independence and into the estimated functional independence after discharge in order to adequately inform patients and relatives, to evaluate progress and to determine the necessity for post-discharge care and assistance (4).
Rehabilitation following SCI focuses on regaining functional independence. Therefore, important aspects of initial SCI rehabilitation are learning new (wheelchair) skills and training in activities of daily living (ADLs). Additionally, during SCI rehabilitation effort is put in the prevention and treatment of complications, which not only contributes to a reduction in morbidity, but may also improve the rehabilitation process and functional independence following SCI (3, 5–8). Another aspect of initial rehabilitation is muscle strength and endurance training. Patients with SCI have a low physical capacity (i.e. the combined function of their muscles, and respiratory and cardiovascular systems is reduced) and their physical capacity can improve (9, 10). However, it is largely unknown whether a certain level of physical capacity is a pre-requisite for functional independence following SCI, and whether the improvement in physical capacity contributes to functional independence.
Other studies have described functional independence following SCI (7, 11, 12), and reported on associations with physical capacity. In these studies patients indicated they felt that inadequate muscle strength and a low endurance capacity threatened their functional independence (13, 14). Additionally, data showed that a low physical capacity was associated with functional limitations (7, 15–17). However, prospective data on changes in functional independence during and after inpatient rehabilitation, and on the associations between changes in functional independence and in physical capacity have not been analysed in a large population. These data are needed to help establish the possible beneficial effect of improving physical capacity in terms of functional independence and health-related functional status. Therefore, this prospective study of a cohort of subjects with SCI at their initial rehabilitation had 3 objectives. The first objective was to determine changes in the level of functional independence over time during and after inpatient rehabilitation. The second objective was to investigate the association between changes in functional independence and changes in physical capacity. The third objective was to determine whether physical capacity at discharge was associated with functional independence and health-related functional status 1 year after discharge.
METHODS
This study was part of the larger Dutch research programme on “Physical strain, work capacity and mechanism of restoration of mobility in the rehabilitation of persons with SCI”. Between July 2000 and November 2005 the research programme included patients with SCI admitted to one of the 8 participating Dutch rehabilitation centres. Patients were eligible to participate if it was their initial rehabilitation for SCI, if they were between 18 and 65 years of age, had tetraplegia or paraplegia American Spinal Injury Association (ASIA) impairment scale A–D, had sufficient comprehension of the Dutch language to understand the purpose of the study and did not have a progressive disease or psychiatric condition that would interfere with constructive participation. Because most patients with SCI require a wheelchair (1), the programme excluded patients who did not use a wheelchair in daily life at initial rehabilitation. This had the advantage that the recovery of functioning could be compared fairly between (or within) subjects with a similar level of functioning. If subjects regained their walking ability during the programme, they did not perform a new maximal graded exercise test with a wheelchair on a treadmill.
Reasons for excluding patients from the maximal graded exercise test were cardiovascular disease (e.g. a recent change in resting electrocardiogram suggesting infarction, complicated myocardial infarction or unstable angina (18), diastolic blood pressure > 90 mmHg or systolic blood pressure > 180 mmHg, musculoskeletal complaints of the arms, neck or trunk, infection with fever or a pressure sore prohibiting sitting (19).
For patients to be included in the study sample, both their functional independence and physical capacity had to be assessed at least once. The protocol was approved by the medical ethics committee, and prior to participation all patients gave their written informed consent.
Procedure
Subjects were assessed on 4 occasions: at the start of active inpatient rehabilitation, defined as the moment when the subject could sit in a wheelchair for at least 3 h at a time (t1), 3 months into active rehabilitation (t2), at discharge (t3), and one year after discharge from inpatient rehabilitation (t4). If subjects were discharged within 3 months after t2, the relatively recent t2 assessment was considered as a discharge (t3) assessment.
Several precautionary measures were taken to optimize the validity and reliability of the data. At each rehabilitation centre, one research assistant was responsible for the data registration according to a standardized protocol. The research assistants were either occupational therapists or physiotherapists with several years of experience in SCI rehabilitation. Prior to and during the programme, they were trained in how to complete the FIM and the SIP68, and how to determine physical capacity in a standardized way. A measurement protocol, which gave clear guidelines for the procedure, was followed. All necessary equipment was calibrated prior to use. The calculation of the peak power output was adjusted as suggested by de Groot et al. (20).
Functional independence and health-related functional status
At each assessment (t1–t4), the Dutch FIM (version 5.0) was completed (7). FIMmotor was calculated by adding the scores of 13 FIM items on mobility and self-care. The SIP68 determined health-related functional status one year after discharge (t4). For each SIP68 item, subjects indicated whether their health condition currently limited this activity (score = 1) or not (score = 0). The physical dimension of the SIP68 was the sum score of items on somatic autonomy and mobility control (range 0–29). The social dimension was the sum score of items on social behaviour and mobility range (range 0–22) (21). The higher the score, the more health-related functional status was affected. Because subjects used a wheelchair, items on mobility were re-coded as recommended by Post et al. (22).
Independent variables
Physical capacity. Physical capacity was established by measuring endurance capacity (peak oxygen uptake and peak power output) and arm muscle strength. We asked subjects not to smoke or drink coffee or alcohol at least 2 h before the tests and to void their bladder before the exercise test started. During 2 blocks of submaximal exercise a suitable treadmill velocity was chosen for the graded maximal exercise test. The submaximal exercise was followed by 2 min of rest, after which the maximal graded exercise test started. Subjects were encouraged to maintain the speed of the treadmill whilst its inclination was increased by 0.36º every min. Finally, the test stopped when the subject was completely exhausted, or could no longer maintain the speed of the treadmill (17, 19). The peak oxygen uptake (l/min) was defined as the highest average oxygen consumption recorded during 30 sec, and the peak power output (W) as the workload at the highest inclination that the subject could maintain for at least 30 sec (23).
Muscle strength was tested in both arms (shoulder abductors, internal and external rotators and elbow flexors and elbow extensors). For those muscle groups with a strength of at least grade 3 during manual muscle testing, strength was determined with handheld dynamometry by using the break-test (24, 25). Subsequently, arm muscle strength was derived from a summation of the strength of these 10 muscle groups (in Newtons) (19). We included those subjects in whom strength was assessed in all arm muscle groups.
Subject characteristics and complications. Age, level and completeness of the lesion and the occurrence of complications, were registered on each occasion. Tetraplegia (score = 0) was defined as a lesion at or above the first thoracic segment, and paraplegia (score = 1) as a lesion below the first thoracic segment. Completeness of the lesion was registered as follows: ASIA category A and B were defined as motor complete (score = 1), and ASIA category C or D were defined as a motor incomplete (score = 0) (26).
A physician used medical charts and self-reported information to determine whether a complication (urinary tract infection, pulmonary infection or pressure sore) had occurred since admission (reported at the start, t1) or since the previous assessment (reported at t2, t3 and t4, respectively), and whether this had resulted in bed rest during this period (8). Either the subject had at least one complication since the previous assessment (score = 1), or the subject had no complications (score = 0). Subsequently, these complications resulted in bed rest (score = 1) or not (score = 0). Self-reported information on musculoskeletal pain in the arms, legs, neck and back was registered using a 5-point Likert scale. These scores were re-coded into the presence of pain (score = 1), or the overall absence of pain (score = 0) (27). Spasticity was assessed at the arms and legs by a fast passive stretch (28). The presence of spasticity (score = 1), or the overall absence of spasticity (score = 0) was registered.
Statistics
Changes in functional independence over time. Random coefficient analyses (MlwiN version 1.1; Centre for Multilevel Modelling, Institute of Education, London, UK) were used to determine changes in functional independence over time, whereby we took into account the repeated assessments within one subject and within one rehabilitation centre (29). A basic model included 3 time-related dummy variables, of which the regression coefficients (with confidence intervals) reflected changes in FIMmotor over time.
Changes in functional independence over time in association with changes in physical capacity. We determined which independent variables were associated with changes in FIMmotor over time. First, all variables at t3 were cross-tabulated, and in case of strong intercorrelations (correlation coefficient > 0.80), the variable with the strongest association with FIMmotor was included for further analyses (30). Peak power output, peak oxygen uptake and arm muscle strength were related to one another (19, 30). Of these parameters, peak power output had the strongest association with FIMmotor, and was therefore included in the multivariate analyses. There were no substantial associations (correlation coefficients < 0.60) between the other independent variables. To determine the significance of each independent variable, first all independent variables (and their interactions with time) were added to the aforementioned basic model one-by-one. A multivariate model was then made for the associations with (changes in) FIMmotor. Therefore, all significant independent variables and interaction terms (p ≤ 0.10) were simultaneously added to the basic model. We narrowed down the consequent multivariate model by alternately removing a non-significant (p > 0.05) independent variable, and re-running the analyses. The regression coefficients in the resultant multivariate model indicated an increase in FIMmotor associated with an increase in the independent variable of one unit (29).
Physical capacity as a predictor of functional independence and health-related functional status. We made multiple regression models to establish whether physical capacity at discharge predicted FIMmotor, and social and physical dimensions of health-related functional status one year after discharge (SPSS version 12.0.01). Because the physical capacity variables were intercorrelated, and peak power output (in Watts) showed a stronger association with both functional independence and health-related functional status, this component of physical capacity was included in the analyses. To determine whether peak power output was of additional value in the prediction of functional independence, we entered the FIMmotor at discharge and then included the other independent variables following the stepwise-forward procedure. In the final multivariate models, the regression coefficients indicated an increase in FIMmotor or in SIP68 associated with an increase in the independent variable of one unit.
RESULTS
Descriptive statistics (SPSS version 12.0.01) summarized subject characteristics at each occasion (Table I). The total study sample included 182 subjects, of whom 176 were assessed at the start (t1), 124 were assessed at 3 months (t2), 160 were assessed at discharge (t3), and 133 were assessed one year after discharge (t4). The size of the sample differed for a variation of reasons (Table II). At the start of active rehabilitation, the mean (standard deviation (SD)) time since injury was 88 (61) days, and at discharge from inpatient rehabilitation, 290 (140) days had passed since injury.
| Table I. Descriptive statistics of subject characteristics and functional independence |
| Variable | Start n = 176 | 3 months n = 124 | Discharge n = 160 | 1 year after discharge n = 133 |
| Age, years* | 40 (14) | 41 (14) | 40 (14) | 41 (14) |
| Men, %* | 76 (133) | 77 (95) | 74 (118) | 72 (96) |
| Paraplegia, % | 69 (121) | 67 (83) | 70 (111) | 74 (97) |
| Motor complete lesion, % † | 67 (117) | 48 (58) | 48 (75) | 53 (68) |
| Peak oxygen uptake, l/min | 1.03 (0.36) | 1.15 (0.42) | 1.22 (0.44) | 1.32 (0.51) |
| Peak power output, W | 31 (18) | 37 (21) | 41 (23) | 48 (25) |
| Arm muscle strength, N | 1547 (533) | 1678 (554) | 1805 (538) | 1864 (608) |
| Complications, % ‡ | 62 (109) | 71 (87) | 52 (82) | 66 (87) |
| Bed rest, % § | 24 (42) | 35 (43) | 17 (27) | 30 (39) |
| Musculoskeletal pain, % | 79 (139) | 77 (95) | 68 (109) | 64 (85) |
| Spasticity, % | 66 (102) | 74 (89) | 68 (104) | 70 (86) |
| FIMmotor¶ | 44 (18) | 58 (20) | 69 (17) | 69 (19) |
| Physical SIP68¶ | n.a. | n.a. | n.a. | 12 (7) |
| Social SIP68¶ | n.a. | n.a. | n.a. | 6 (4) |
| *Mean (standard deviation) or percentage of study sample (n) is given. †Motor complete: American Spinal Injury Association (ASIA) category A or B. ‡Complications: those who had at least one complication since admission (reported at the start of active rehabilitation) or since previous occasion. §Bed rest: those who had bed rest for these complications. ¶FIMmotor: level of functional independence (range 13–91); physical SIP68: physical dimension of Sickness Impact Profile 68 (range 0–29); social SIP68: social dimension of SIP68 (range 0–22); n.a.: not applicable, because not determined at these occasions. |
| Table II. Reasons for exclusion from the study sample on a specific occasion |
| | Start | 3 months | Discharge | 1 year after discharge |
| Included in study sample | 176 | 124 | 160 | 133 |
| Corset | 3 | n.a. | n.a. | n.a. |
| Complications or contra-indications* | 3 | 3 | 1 | 4 |
| Discharged† | n.a. | 41 | n.a. | n.a. |
| Refusal or not reached | n.a. | 1 | 2 | 16 |
| Walking‡ | n.a. | 4 | 5 | 4 |
| Dead | n.a. | 1 | 1 | 3 |
| Technical limitations§ | 2 | 2 | 3 | n.a. |
| *Complications or contra-indications to tests that determine physical capacity. †For those who were discharged soon after t2 (3 months), the t2 assessment was considered a discharge (t3) assessment. ‡Those who could walk did not perform another exercise test on a wheelchair treadmill. §Technical limitations were problems that prohibited the collection of data on physical capacity on this occasion. n.a.: not applicable. |
Changes in functional independence over time
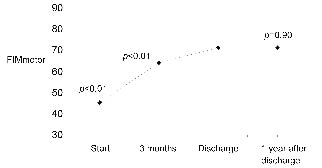
Fig. 1 illustrates changes in FIMmotor over time, as estimated with the basic multi-level random coefficient model. It shows that the FIMmotor score increased during rehabilitation, but did not change significantly after discharge from inpatient rehabilitation.
Fig. 1. Functional Independence Measure (FIMmotor) during and after inpatient rehabilitation. p-values for significance of the difference between FIMmotor on this occasion and that at discharge as estimated using the basic multi-level random coefficient model.
Changes in functional independence over time in association with changes in physical capacity
Table III gives the individual associations between functional independence and the separate independent variables, which were added to the basic model. The FIMmotor was associated with peak oxygen uptake, peak power output, muscle strength and complications. The final multivariate multi-level model showed a positive association with peak power output and a negative association with a tetraplegia and the occurrence of complications (Table IV). For example, an increase in peak power output of 10 Watts was associated with an increase in FIMmotor of nearly 4 points (10 Watts × 0.36). The significant interaction term indicated that the association with peak power output changed over time.
| Table III. Individual associations with Functional Independence Measure (FIMmotor score) |
| Independent variable | Regression coefficient | Confidence interval |
| Peak oxygen uptake, l/min | 14.3 | 10.6 to 18.1 |
| Peak power output, W | 0.40 | 0.32 to 0.48 |
| Muscle strength, N | 0.02 | 0.01 to 0.02 |
| Complications (1: present; 0: absent) | –2.15 | –4.25 to –0.05 |
| Bed rest (1: present; 0: absent) | –0.04 | –2.31 to 2.23 |
| Spasticity (1: present; 0: absent) | –1.44 | –4.44 to 1.56 |
| Musculoskeletal pain (1: present; 0: absent) | –1.08 | –3.37 to 1.21 |
| Gender (1: men; 0: women) | 5.23 | –10.0 to 20.5 |
| Age (years) | –0.09 | –0.25 to 0.07 |
| Level (1: paraplegia; 0: tetraplegia) | 9.04 | 6.69 to 11.4 |
| Motor completeness (1: complete; 0: incomplete) | –0.97 | –3.64 to 1.70 |
| Independent variables were added separately to the basic random coefficient model. Significant associations are shown in bold. |
Physical capacity as a predictor of functional independence and health-related functional status 1 year after discharge
The multiple regression models showed that peak power output made a modest but significant contribution to the prediction of outcome one year after discharge (Tables V and VI). Together, discharge FIMmotor and peak power output predicted a large proportion of the variance (85%) in FIMmotor one year after discharge (Table V). Taking into account the FIMmotor at discharge, the peak power output at discharge significantly predicted the social dimension of SIP68 (R2 = 18%). The FIMmotor at discharge and completeness of the lesion significantly contributed to the prediction of the physical dimension of the SIP68 (R2 = 38%) (Table VI). The residual standard deviations (RSD) indicate the prediction accuracy of the model, i.e. whether the predicted outcome corresponds with the observed outcome.
| Table IV. Functional Independence Measure (FIMmotor) in association with physical capacity: multivariate multi-level random coefficient analyses |
| Variables in model | Regression coefficient* | 95% confidence interval | p-value |
| Change over time | | | |
| Discharge; t3 | 58.82 | 53.02 to 64.62 | < 0.01 |
| Start minus discharge; t1 – t3 (0/1) | –22.18 | –27.16 to –17.20 | < 0.01 |
| 3 months minus discharge; t2 – t3 (0/1) | –12.20 | –17.30 to –7.10 | < 0.01 |
| 1 year post-discharge minus discharge; t4 – t3 (0/1) | 2.40 | –2.93 to 7.73 | 0.37 |
| Independent variable | | | |
| Peak power output (W) | 0.36 | 0.20 to 0.46 | < 0.01 |
| Peak power output t1–t3* | 0.06 | –0.06 to 0.18 | 0.32 |
| Peak power output t2–t3* | 0.20 | 0.08 to 0.32 | < 0.01 |
| Peak power output t4–t3* | –0.03 | –0.13 to 0.07 | 0.55 |
| Complications (1: present; 0: absent) | –2.70 | –4.93 to –0.47 | 0.02 |
| Interaction terms are linked by asterisks (*). |
| Table VI. Predictive models for physical and social dimension of the Sickness Impact Profile (SIP68) one year after discharge: multiple linear regression analyses (n = 102) |
| Variable at discharge | Physical dimension | Social dimension |
| Regression coefficient (SE) | Variance* | p-value | Regression coefficient (SE) | Variance | p-value |
| Constant | 25.92 | n.a. | n.a. | 11.89 | n.a. | n.a. |
| FIMmotor at discharge | –0.23 | 34% | < 0.000 | –0.06 | 13% | 0.080 |
| Peak power output, W | n.e. | n.e. | n.e. | –0.06 | 5% | 0.010 |
| Motor completeness (0: incomplete; 1: complete) | 2.42 | 4% | 0.012 | n.e. | n.e. | n.e. |
| Accuracy | R2 = 0.38; RSD = 4.74 | R2 = 0.18; RSD = 3.88 |
| Adjusted R2 and residual standard deviation (RSD) indicate the accuracy of the model. *Percentage of variance explained by this independent variable. n.e.: variable not modelled because not significant during stepwise forward procedure; n.a.: not applicable; SE: standard error. |
| Table V. Predictive models for Functional Independence Measure (FIMmotor) one year after discharge: multiple regression analyses (n = 103) |
| Predictors in model | Regression coefficient (SE) | p-value* |
| Constant | 1.65 (3.17) | n.a. |
| FIMmotor at discharge | 0.95 (0.05) | < 0.001 |
| Peak power output, W | 0.07 (0.04) | 0.046 |
| Accuracy model | R2 = 0.85; RSD = 7 |
| *p-value for the significance of the (additional) value of this independent variable in the prediction of FIMmotor 1 year after discharge. Adjusted R2 and residual standard deviation (RSD) indicate the accuracy of the model. n.a.: not applicable; SE: standard error. |
DISCUSSION
Changes in functional independence over time
This prospective study of subjects with SCI showed that functional independence improved during inpatient rehabilitation, but stabilized after discharge. This unaltered FIMmotor after discharge is in agreement with other studies and may be explained by several factors (7, 31, 32). First, the FIMmotor only determines functional independence during basic ADL (32, 33). For some patients, higher levels of activities and participation may need to be measured in order to be able to detect recovery. Secondly, in the Netherlands the duration of rehabilitation is relatively long (7). During this intense and long inpatient rehabilitation patients acquire different skills (3, 7). Fortunately, our results suggest that the environment outside the rehabilitation centre has not limited these patients in putting their skills into practice.
To further investigate changes in FIMmotor, we compared the recovery of FIMmotor during one year after discharge in different groups. This showed that functional independence in subjects in the higher deciles of discharge FIMmotor did not change, those in the middle deciles improved, and those in the lower deciles worsened. These differences in recovery may be another reason why, overall, the FIMmotor did not change. A decline in functional independence in the already poorly performing subjects could perhaps be caused by the fact that a patient’s ability to do something does not necessarily coincide with his or her behaviour (34, 35). For example, a patient may have learnt the skills to carry out an activity, but may choose to be assisted in order to be able to spend his energy on other activities. These choices may negatively affect the measured level of FIMmotor, but being able to make these choices contributes to a sense of control and wellbeing (2, 14). Therefore, we recommend that rehabilitation professionals strive for the optimal development of skills, because this allows patients to make a choice.
Changes in functional independence over time in association with changes in physical capacity
In agreement with the cross-sectional associations, changes in physical capacity were associated with changes in functional independence (15, 17). Because we included wheelchair users, the endurance capacity determined on a wheelchair treadmill was expected to be associated with functional independence (15). The peak power output was more strongly associated with independence than was peak oxygen uptake and muscle strength, probably because it is a more comprehensive measure of body function, more closely related to activities and influenced by the technique of wheelchair propulsion (17, 36). Furthermore, the performance of transfers necessary for ADLs are of short duration, which probably make these activities more strongly associated with peak power output than with peak oxygen uptake (17, 36).
Surprisingly, the peak power output proved more important in the multivariate models than characteristics such as gender and level of the lesion. Obviously, with the dichotomization of level of the lesion, information is lost, and it is conceivable that if we had modelled more detailed lesion characteristics we may have found different associations. However, because we included only subjects who used a wheelchair, and in whom we were able to determine physical capacity, the lesion groups were more homogeneous, and the influence of these lesion characteristics may not be as large as expected. We hypothesize that subject and lesion characteristics are embodied in the peak power output, and that the influence of skills and technique make the power output especially associated with activities in wheelchair users. Therefore, because of its consistent association with functional independence, we recommend the regular assessment of peak power output as an objective measure of rehabilitation outcome in patients with SCI who use a wheelchair.
The negative association between complications and functional independence found in our prospective study coincides with cross-sectional data reported by others (16, 37). Although bed rest may result in deconditioning, it was not associated with an expected functional decline in our study sample (7, 38). Therefore, bed rest may have caused subjects temporarily to carry out fewer activities, but did not affect functional independence. Spasticity and pain have different consequences in different situations, which is probably why we did not find them to be associated with functional independence (27, 37). For example, whilst spasticity may limit arm function, it may be useful in the legs when making a transfer (37). Similarly, patients who are active may be exposed to overuse and, therefore, susceptible to pain. Conversely, pain may, in turn, limit activities (39). Results seem to suggest that in the presence of complications, clinicians and patients need to be aware of concomitant changes in functional independence.
Physical capacity as a predictor of functional independence and health-related functional status one year after discharge
The additional predictive value of peak power output seems to suggest that not only functional training, but also enhancing physical capacity, could contribute to the recovery of functional independence. The positive association between peak power output and the social dimension of SIP68 is consistent with a previous study (15), and may be explained by the positive association between peak power output and wheelchair skills: if peak power output enhances wheelchair skills, it may indirectly improve social activities (15, 40).
Limitations
The selection of our study sample needs to be considered when interpreting results. At the start, we included wheelchair users only, which meant that a relatively large proportion of subjects with a complete lesion were selected (41). Furthermore, we included only those subjects whose level of physical capacity could be established, which means that relatively poor performing subjects were excluded. This selection could explain why the association with level or completeness of the lesion was perhaps not as strong as expected (7, 16, 31). However, because our study sample included relatively many women, many patients with a tetraplegia, patients with either a traumatic or non-traumatic lesion and many patients who were only moderately active, our data are more characteristic of patients with SCI than the study samples described in many previous reports on (the influence of) physical capacity following SCI (42).
The outcome measures may need some consideration. Although the SCI may also influence emotional and psychological function, we did not investigate psychological, communicative and emotional items of the FIM or SIP68, because these are considered to be more stable and less informative following SCI (22, 32). This study did not determine changes in health-related functional status. However, health-related functional status is probably especially informative after discharge, when patients face different restrictions in participation. Therefore, we recommend that health-related functional status and its determinants be investigated in future follow-up studies of patients with SCI.
Although associations were consistent, one has to consider that the peak power output made a modest contribution to the variance in outcome at follow-up, and that the prediction accuracy of the models was relatively poor (29, 30). Cross-validation in a different population may show an even less promising predictive value (30, 43). Therefore, intervention studies need to investigate whether training physical capacity improves functional independence.
In conclusion, functional independence improves during inpatient rehabilitation, but remains unchanged thereafter. Peak power output is positively associated with functional independence. The additional value of peak power output suggests that both functional training and enhancing physical capacity could contribute to functional independence and health-related functional status. However, future intervention studies are needed to confirm these proposed beneficial effects.
AcknowledgEment
This project was supported by The Health Research and Development Council of The Netherlands (grant no. 1435.0003; 1435.0025).
REFERENCES
1. Post MW, van Asbeck FW, van Dijk AJ, Schrijvers AJ. Services for spinal cord injured: availability and satisfaction. Spinal Cord 1997; 35: 109–115.
2. Whalley Hammell K. Experience of rehabilitation findings following spinal cord injury: a meta-synthesis of qualitative findings. Spinal Cord 2007; 45: 260–274.
3. van Asbeck FWA, editor. Handboek dwarslaesierevalidatie [Handbook on spinal cord injury rehabilitation]. 2nd edn. Houten: Bohn Stafleu van Loghum; 2007 (in Dutch).
4. Tooth L, McKenna K, Geraghty T. Rehabilitation outcomes in traumatic spinal cord injury in Australia: functional status, length of stay and discharge setting. Spinal Cord 2003; 41: 220–230.
5. Bloemen-Vrencken JH, Post MW, Hendriks JM, De Reus EC, De Witte LP. Health problems of persons with spinal cord injury living in the Netherlands. Disabil Rehabil 2005; 27: 1381–1389.
6. Noreau L, Proulx P, Gagnon L, Drolet M, Laramee MT. Secondary impairments after spinal cord injury: a population-based study. Am J Phys Med Rehabil 2000; 79: 526–535.
7. Post MW, Dallmeijer AJ, Angenot EL, van Asbeck FW, van der Woude LH. Duration and functional outcome of spinal cord injury rehabilitation in the Netherlands. J Rehabil Res Dev 2005; 42: 75–85.
8. Haisma JA, van der Woude LH, Stam HJ, Bergen MP, Sluis TA, Post MW, et al. Complications following spinal cord injury: occurrence and risk factors in a longitudinal study during and after inpatient rehabilitation. J Rehabil Med 2007; 39: 393–398.
9. Dallmeijer AJ, van der Woude LH, Hollander AP, van As HH. Physical performance during rehabilitation in persons with spinal cord injuries. Med Sci Sports Exerc 1999; 31: 1330–1335.
10. Stewart MW, Melton-Rogers SL, Morrison S, Figoni SF. The measurement properties of fitness measures and health status for persons with spinal cord injuries. Arch Phys Med Rehabil 2000; 81: 394–400.
11. Dahlberg A, Kotila M, Kautiainen H, Alaranta H. Functional independence in persons with spinal cord injury in Helsinki. J Rehabil Med 2003; 35: 217–220.
12. Lugo LH, Salinas F, Garcia HI. Out-patient rehabilitation programme for spinal cord injured patients: evaluation of the results on motor FIM score. Disabil Rehabil 2007; 29: 873–881.
13. Price GL, Kendall M, Amsters DI, Pershouse KJ. Perceived causes of change in function and quality of life for people with long duration spinal cord injury. Clin Rehabil 2004; 18: 164–171.
14. Carpenter C, Forwell SJ, Jongbloed LE, Backman CL. Community participation after spinal cord injury. Arch Phys Med Rehabil 2007; 88: 427–433.
15. Dallmeijer AJ, van der Woude LH. Health related functional status in men with spinal cord injury: relationship with lesion level and endurance capacity. Spinal Cord 2001; 39: 577–583.
16. Post MW, de Witte LP, van Asbeck FW, van Dijk AJ, Schrijvers AJ. Predictors of health status and life satisfaction in spinal cord injury. Arch Phys Med Rehabil 1998; 79: 395–401.
17. Kilkens OJ, Dallmeijer AJ, de Witte LP, van der Woude LH, Post MW. The Wheelchair Circuit: Construct validity and responsiveness of a test to assess manual wheelchair mobility in persons with spinal cord injury. Arch Phys Med Rehabil 2004; 85: 424–431.
18. American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 6th edn. Philadelphia: Lippincott, William & Wilkins; 2000.
19. Haisma JA, Bussmann JB, Stam HJ, Sluis TA, Bergen MP, Dallmeijer AJ, et al. Changes in physical capacity during and after inpatient rehabilitation in subjects with a spinal cord injury. Arch Phys Med Rehabil 2006; 87: 741–748.
20. de Groot S, Zuidgeest M, van der Woude LH. Standardization of measuring power output during wheelchair propulsion on a treadmill: pitfalls in a multi-center study. Med Eng Phys 2006; 28: 604–612.
21. Nanda U, McLendon PM, Andresen EM, Armbrecht E. The SIP68: an abbreviated sickness impact profile for disability outcomes research. Qual Life Res 2003; 12: 583–595.
22. Post MW, de Bruin A, de Witte L, Schrijvers A. The SIP68: a measure of health-related functional status in rehabilitation medicine. Arch Phys Med Rehabil 1996; 77: 440–445.
23. van der Woude LH, de Groot G, Hollander AP, van Ingen Schenau GJ, Rozendal RH. Wheelchair ergonomics and physiological testing of prototypes. Ergonomics 1986; 29: 1561–1573.
24. Phillips BA, Lo SK, Mastaglia FL. Muscle force measured using “break” testing with a hand-held myometer in normal subjects aged 20 to 69 years. Arch Phys Med Rehabil 2000; 81: 653–661.
25. Sisto SA, Dyson-Hudson T. Dynamometry testing in spinal cord injury. J Rehabil Res Dev 2007; 44: 123–136.
26. Maynard FM Jr, Bracken MB, Creasey G, Ditunno JF Jr, Donovan WH, Ducker TB, et al. International standards for neurological and functional classification of spinal cord injury. American Spinal Injury Association. Spinal Cord 1997; 35: 266–274.
27. Burchiel KJ, Hsu FP. Pain and spasticity after spinal cord injury: mechanisms and treatment. Spine 2001; 26: S146–S160.
28. Lance JW. Spasticity: disordered motor control. In: Feldman RG, Young RR, Koella WP, editors. Symposium synopsis. Chicago: Year Book Medical Publishers; 1980, p. 485–495.
29. Twisk JW, editor. Applied longitudinal data analysis for epidemiology; a practical guide. Cambridge: Cambridge University Press; 2003.
30. Field A. Regression. In: Wright DB, editor. Discovering statistics: using SPSS for Windows. London: Sage Publications Ltd; 2000, p. 102–162.
31. Fisher CG, Noonan VK, Smith DE, Wing PC, Dvorak MF, Kwon BK. Motor recovery, functional status, and health-related quality of life in patients with complete spinal cord injuries. Spine 2005; 30: 2200–2207.
32. Hall KM, Cohen ME, Wright J, Call M, Werner P. Characteristics of the Functional Independence Measure in traumatic spinal cord injury. Arch Phys Med Rehabil 1999; 80: 1471–1476.
33. Middleton JW, Harvey LA, Batty J, Cameron I, Quirk R, Winstanley J. Five additional mobility and locomotor items to improve responsiveness of the FIM in wheelchair-dependent individuals with spinal cord injury. Spinal Cord 2006; 44: 495–504.
34. World Health Organization. International Classification of Functioning, Disability and Health: ICF. In. Geneva: World Health Organization; 2001.
35. Marino RJ. Domains of outcomes in spinal cord injury for clinical trials to improve neurological function. J Rehabil Res Dev 2007; 44: 113–122.
36. van der Woude LH, Bouten C, Veeger HE, Gwinn T. Aerobic work capacity in elite wheelchair athletes: a cross-sectional analysis. Am J Phys Med Rehabil 2002; 81: 261–271.
37. Dvorak MF, Fisher CG, Hoekema J, Boyd M, Noonan V, Wing PC, et al. Factors predicting motor recovery and functional outcome after traumatic central cord syndrome: a long-term follow-up. Spine 2005; 30: 2303–2311.
38. Haisma JA, Bussmann JB, Stam HJ, Sluis TA, Bergen MP, Post MW, et al. Physical fitness in persons with a spinal cord injury: the association with complications and duration of rehabilitation. Clin Rehabil 2007; 21: 932–940.
39. van Drongelen S, de Groot S, Veeger HE, Angenot EL, Dallmeijer AJ, Post MW, et al. Upper extremity musculoskeletal pain during and after rehabilitation in wheelchair-using persons with a spinal cord injury. Spinal Cord 2006; 44: 152–159.
40. Kilkens OJ, Dallmeijer AJ, Nene AV, Post MW, van der Woude LH. The longitudinal relation between physical capacity and wheelchair skill performance during inpatient rehabilitation of people with spinal cord injury. Arch Phys Med Rehabil 2005; 86: 1575–1581.
41. de Groot S, Dallmeijer AJ, Post MW, van Asbeck FW, Nene AV, Angenot EL, et al. Demographics of the Dutch multicenter prospective cohort study “Restoration of mobility in spinal cord injury rehabilitation”. Spinal Cord 2006; 44: 668–675.
42. Haisma JA, van der Woude LH, Stam HJ, Bergen MP, Sluis TA, Bussmann JB. Physical capacity in wheelchair-dependent persons with a spinal cord injury: a critical review of the literature. Spinal Cord 2006; 44: 642–652.
43. Altman DG, Royston P. What do we mean by validating a prognostic model? Stat Med 2000; 19: 453–473.