OBJECTIVE: To evaluate end-point acuity in goal-directed arm movements in subjects with chronic neck pain, while taking the trade-off between speed and accuracy into account, and to evaluate associations between reduced acuity and self-rated characteristics.
DESIGN: Single-blinded, controlled, comparative group study.
SUBJECTS: Forty-five subjects with chronic non-traumatic, non-specific neck pain (n = 24) and whiplash-associated disorders (n = 21). Healthy subjects served as controls (n = 22). The groups were age- and sex-matched.
METHODS: Subjects performed fast and accurate pointing movements to a visual target. Group differences in end-point variability, controlled for peak velocity, were evaluated. Associations between end-point variability and self-rated symptoms, functioning, self-efficacy and kinesiophobia were analysed.
RESULTS: End-point acuity, controlled for peak velocity, was reduced for both neck-pain groups. Similar spatial error patterns across all groups indicated no direction-specific reduction. For both neck-pain groups, associations were found between end-point acuity and neck movement deficits, physical functioning and, in whiplash, also balance and pain.
CONCLUSION: Acuity of goal-directed arm movements can be reduced in chronic neck pain. Associations between acuity and self-rated characteristics support the clinical validity of the results and indicate that impaired neck function contributes to reduced end-point acuity. The results can be of importance for characterization and rehabilitation of neck disorders.
Key words: neck pain, whiplash injuries, somatosensory disorders, motor activity, psychomotor performance, proprioception, kinaesthesis, vision.
J Rehabil Med 2008; 40: 366–374
Correspondence address: Mats Djupsjöbacka, Centre for Musculoskeletal Research, University of Gävle, Box 7629, SE-907 12 Umeå, Sweden. E-mail: mda@hig.se
Submitted July 17, 2007; accepted December 18, 2007
*This paper was presented as a poster at the International Association for the Study of Pain (IASP) symposium “Fundamentals of musculoskeletal pain”, on May 7–9, 2007 in Aalborg, Denmark and the PREMUS conference, on August 27–30, 2007 in Boston, USA.
Introduction
Subjects who present with chronic neck pain often exhibit atypical postures and movement patterns that are obvious to many clinicians. With the development of modern methods for objective assessment of movement kinematics and dynamics, other, less apparent, alterations in sensorimotor control associated with neck pain have become evident. Evidence of such alterations includes, for example, altered recruitment patterns of cervical muscles (1), poor balance (2, 3) and reduced acuity of cervical proprioception (4, 5). On the basis of such findings, along with data from experimental models, alterations in sensorimotor function have been suggested to play a significant role in the pathogenesis and maintenance of chronic neck pain (1, 6).
Recent studies have also shown that chronic neck pain can be associated with reduced proprioceptive acuity in the elbow and shoulder joints. Knox et al. (7) showed that elbow joint position error was increased to a greater extent by changes in head and neck position in subjects with chronic whiplash-associated disorders (WAD) compared with healthy controls. Similarly, we found a reduced repositioning acuity of the shoulder in subjects with traumatic neck pain (8). These studies used single-joint ipsilateral position matching under blindfolded conditions to test repositioning acuity. In spite of this rather unnatural testing condition, the results from our laboratory revealed significant associations between the matching acuity and self-ratings of physical functioning and the ability to perform everyday tasks (8, Djupsjöbacka et al.1).
1Djupsjöbacka M, Sandlund J, Röijezon U, Björklund M. Shoulder proprioception in chronic neck pain. Associations with symptoms and self-related characteristics, in preparation.
Everyday tasks normally require interaction with the environment involving control of multi-joint movements in 3-dimensional space. Recent research has shown that the control of this type of movement relies on both visual and proprioceptive feedback (9). The fact that the control of 3-dimensional multi-joint movements relies on proprioceptive feedback, together with the findings of impaired repositioning acuity of the shoulder in chronic neck pain (8, Djupsjöbacka et al.1), implies that neck pain may also be associated with reduced acuity of reaching movements. Surprisingly, to our knowledge, this has not been studied, although reaching movements are a key component of many everyday activities. Thus, the present study intends to extend our previous research on shoulder repositioning acuity by studying end-point acuity in fast pointing (multi-joint) movements in chronic neck pain.
Woodworth (10) was one of the first to describe the somewhat intuitive speed-accuracy trade-off in movement control: the faster we move, the less precise are our movements, and vice versa: the more severe the constraints are, the slower we move. This relationship was later summarized by Paul Fitts in a well-known model for rapid aimed movements; Fitts’ Law (11). Since its introduction the principles of Fitts’ Law have been successfully applied and extended into various domains (12). The speed-accuracy trade-off has been suggested to depend on the fact that faster movements provide less time to process sensory feedback and hence correct the movement, and the increased motor noise from the process of generating the movement (12). This implies that movement speed should be considered when assessing the acuity of goal-directed reaching movements.
As mentioned above, the control of reaching movements seems to rely on both vision and proprioception. One model of how the central nervous system (CNS) integrates vision and proprioception to optimize motor control concerns a direction-dependent weighting of the sensory information (9, 13). Interestingly, it was demonstrated that vision appears to dominate movement control in the horizontal (left-right) direction, whereas proprioception weights more heavily than vision in the depth (near-far) direction (9, 13); a finding that challenges the classical view that the brain always relies more on vision. Theoretically, the increased proprioceptive weight in the depth direction is explained by difficulties of the visual system to make near-far judgments, since target depth has to be derived from relatively less precise estimates such as gaze vergence and disparity. Analyses of the spatial structure of the variability of reaching movements hence provide an opportunity for studying the mechanisms behind impaired reaching acuity.
When studying possible impairments in sensorimotor functions from a rehabilitation perspective, it is important also to investigate associations between these functions and symptoms as well as self-rated functioning and other self-rated characteristics. Such analyses can reveal the clinical relevance of the sensorimotor impairments as well as provide leads to the mechanisms behind the impairments. Here we attempt to apply partial least squares (PLS) regression (14, 15) for investigating the associations between end-point acuity and symptoms as well as self-rated characteristics.
The first hypothesis of the present study was that subjects suffering from chronic neck pain with traumatic as well as non-traumatic aetiology have reduced end-point acuity (i.e. increased end-point variability) in goal-directed pointing movements, when taking the speed-accuracy trade-off into account. A second hypothesis was that; if the hypothesized reduction in end-point acuity is restricted to a proprioceptive deficit, this would, according to the model of direction-specific sensory efficiency described above (9, 13), be reflected in high variability primarily in the near-far (depth) direction when performing pointing movements to a visual target. However, although the clinical picture may differ between traumatic and non-traumatic neck-pain subjects, we do not expect any substantial group differences in end-point variability, and comparisons are, therefore, limited to pre-planned contrast of each of the neck-pain groups and a control group.
The primary aim of the present study was to test whether subjects with chronic neck pain have higher end-point variability in goal-directed pointing movements than healthy controls, taking the speed-accuracy trade-off into account (hypothesis 1). Additional aims were to test whether possible group differences in end-point variability are greater in the near-far direction than in other directions (hypothesis 2), and to study associations between the magnitude of end-point variability and symptoms and self-rated functioning in subjects with chronic neck pain.
METHODS
The study was designed as a single-blinded, controlled and comparative group study. It was performed at a vocational rehabilitation centre (Alfta Rehab Center, Alfta, Sweden). The study was approved by the Regional Ethical Review Board in Uppsala and complies with current Swedish legislation.
Subjects
Forty-five subjects with chronic neck pain, with or without traumatic association, were included in the study. The subjects with neck pain without traumatic association are referred to as “non-specific” (NS, n = 24), whereas the subjects with neck pain associated with trauma to the head or neck are referred to as WAD (n = 21). They were recruited from Alfta Rehab Center, from general practitioners and physiotherapists in the community and by advertising in local papers. To be included they had to have pain in the neck, validated by pain drawings according to Margolis et al. (16), of at least 3 months’ duration and score > 10 on the Neck Disability Index (NDI) (see below). Although non-specific chronic neck pain may comprise specific sub-groups, we made no attempt to divide this group further, since at present, there is little evidence to allow further sub-categorization (see, for example, (17)). To be included in the WAD group the subjects should relate the onset of symptoms to an accident, and the symptoms should have presented within 2 weeks after this accident. Since the medical records from the acute stage of these patients were unavailable, a further subdivision of WAD was not done. Further characteristics of the groups, including questionnaire scores representing general health, functioning and disability, pain ratings and distribution are given in Table I.
| Table I. Characteristics of the study sample. The NDI, DASH and TSK scores are normalized to the range of 0–100 |
| Characteristics | CON (n = 22) | NS (n = 24) | WAD (n = 21) |
| Women, n | 13 | 14 | 11 |
| Men, n | 9 | 10 | 10 |
| Age, mean (SD) (years) | 37 ± 10 | 37 ± 9 | 36 ± 5 |
| BMI, mean (SD) | 25 ± 3 | 26 ± 4 | 26 ± 4 |
| SF-36 PCS, mean (SD) | 55 ± 5 | 41 ± 11* | 34 ± 8* |
| SF-36 MCS, mean (SD) | 52 ± 8 | 41 ± 12* | 36 ± 14* |
| Symptom duration weeks, median, range | NA | 60 (12–368) | 73 (22–215) |
| VAS pain, mean (SD) | NA | 47 ± 23 | 60 ± 22 |
| NDI, mean (SD) | NA | 30 ± 13 | 45 ± 16 |
| DASH, mean (SD) | NA | 20 ± 12 | 34 ± 18 |
| TSK, mean (SD) | NA | 37 ± 15 | 46 ± 14 |
| Pain in shoulders, n | NA | 22 | 19 (19) |
| Pain in upper arms, n | NA | 11 | 9 (19) |
| Pain in lower arms, n | NA | 14 | 9 (19) |
| Pain in hands, n | NA | 14 | 9 (19) |
| *p < 0.05 (Dunnett’s t two-sided post-hoc t-test with the control group). Pain in shoulders, upper arms, lower arms and hands represents the number of subjects indicating pain in these areas for at least one side. Note that n = 19 for the WAD group since 2 pain drawings could not be scored according to the criteria by Margolis et al. (16). BMI: body mass index; SF-36 PCS: Short Form-36 physical component summary; SF-36 MCS: Short Form-36 mental component summary; VAS pain: visual analogue scale rating of pain; NDI: Neck Disability Index; DASH: Disability of the Arm, Shoulder and Hand; TSK: the TAMPA Scale of Kinesiophobia; CON: control, NS: non-specific and WAD: whiplash-associated disorders; SD: standard deviation; NA: not analysed. |
A control group of age- and sex-matched subjects (CON, n = 22) was recruited by advertising in local papers. Control subjects were included if they had no history of head, neck or shoulder trauma and no current neck or shoulder pain or longer periods of constant or intermittent neck-shoulder pain. All subject had to be right-handed and 20–50 years of age. Exclusion criteria for all groups were surgery of the neck, shoulder or back, reported injuries with fractures or luxations to the neck or shoulders, conditions of neurological or rheumatic disease (rheumatoid arthritis, pelvospondylitis) or fibromyalgia. All subjects also had to be able to perform voluntary movements including arm elevations above 110 degrees and at least 25 degrees axial rotation of the head. This was assessed by the physiotherapist conducting the test of end-point acuity (see below). All subjects gave their written consent to participate after being informed about the aims and methods of the study.
Test of end-point acuity in goal-directed pointing
The assessment of end-position acuity in pointing was included as one of 8 sensorimotor tests that were carried out over a period of 2 h on the same test occasion. The test order was the same for all subjects and the pointing acuity test was the second in order. All subjects were tested by the same experimenter. Results from the other tests will be reported elsewhere.
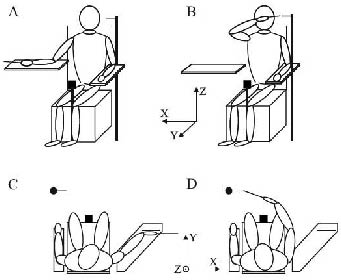
Test set-up. The test set-up is illustrated in Fig. 1. The subjects sat in a rigid chair with their torso strapped with a belt to the back of the chair in order to restrict movements of the torso but allow free movements of the shoulder. A height-adjustable arm support was placed at waist height on the right side of the chair. An adjustable rim was placed parallel to the frontal plane on the arm rest as a guide for placing the hand in the starting position (Fig. 1C). A wooden pointer fixated to a rectangular plastic plate was firmly attached to the hand (total weight 160 g). The pointer was fixed in line with the third digit, extending 20 cm from the fingertip. The plastic plate was attached to the palm of the hand and the fingers in order to keep the fingers extended and prevent movements of the joints distal to the wrist. This arrangement was used in order to maximize the impact from arm proprioception on pointing acuity while minimizing the influence from fine motor control of the hand. The target consisted of a soft foam-rubber stick, 1 cm in diameter, that was placed in front of the subject at a distance corresponding to the location of the wrist of the subjects’ extended arm at eye level height and 20 cm to the left of the subjects left acromion. The target pointed medially to the right parallel to the frontal plane (Fig. 1B, D). The test procedure and data collection was fully automated and computer-controlled and all instructions were pre-recorded and presented through speakers.
Testing procedure. Before the test started the subject was given the following instructions; “The task is to place the pointer as close as possible to the target. You should do this as fast and accurately as possible. When you have reached the target, keep the pointer still for a few seconds and do not correct the position”. The starting position was attained by placing the right hand with the plastic plate against the rim and the lower arm and hand resting on the arm support with the ulna side facing down and with the wrist near full dorsal extension (Fig. 1A, C). The command “Go” indicated that the subject should start the pointing movement. After holding the pointer still for 1 sec at the target (Fig. 1B, D) the subject was instructed to “go to the starting position”. In total, 15 such trials were performed and the subjects were allowed full vision throughout the entire test. Before the test 3–5 practice trials were performed to familiarize with the task and to update the body representation of the elongated end-effector (hand-pointer), which is a process that in manual pointing with long tools has been shown to be instantaneous (18).
Data collection and processing. Kinematic data were recorded with an electromagnetic tracking system (FASTRAK, Polhemus Inc., USA) at a sampling rate of 30 Hz. The magnetic field transmitter was placed on a platform located between the subjects’ knees (see Fig. 1). The global coordinate system had an orientation such that its coordinate axes X, Y and Z, respectively, corresponded to the horizontal, depth and vertical directions in relation to the body (Fig. 1). Pointer coordinates were calculated from data collected by a sensor attached to the hand plate. Since the hand plate and pointer constituted a rigid body, the pointer tip location could be calculated from the sensor data. By holding the pointer tip up against the target tip the spatial location of the target was determined before commencing the test for each subject. Movement initiation and termination were assessed from the velocity profiles of the pointer tip. Movement initiation was defined as the instant when the velocity of the pointer tip exceeded 10% of its maximal value. Movement termination was defined as the moment when the velocity dropped below 10% plus an additional 500 ms. This criterion ensured that the tip remained virtually stationary (19).
As outcome measure for end-point acuity we used the Variable Error (VE) of the pointer tip position at the time of movement termination, calculated separately along each coordinate axis (X, Y and Z, see above). VE was calculated as the population standard deviation of the algebraic errors (distance between pointer and target) for the 15 trials after “detrending” the data in each subject’s test series to remove possible drift in bias, which is unrelated to the response variability but will affect VE (20). The velocity of the pointer tip was computed as the first derivative of the coordinate data with application of a low pass 4th-order Butterworth filter with a cut-off frequency of 3 Hz. The subjects’ peak velocities and movement times, averaged over all 15, trials were used in the analyses.
Fig. 1. The pointing movement task for (A and C) starting and (B and D) target position. The axes in the centre illustrate the orientation of the laboratory coordinate system.
Pain measurement and questionnaires
Within a week before the day of testing, “pain right now” was assessed on a blank 100-mm visual analogue scale (VAS), where 0 mm correspond to “no pain at all” and 100 mm to “worst imaginable pain” (21).
The week before the tests the neck-pain subjects received a number of questionnaires that they completed at home. The questionnaires addressed symptoms, health-related quality of life, pain-related disability, functional self-efficacy, physical functioning and kinesiophobia.
Short Form Health Survey 36. The Short Form Health Survey, SF-36 was used as a measure of general health and well-being (22). The SF-36 provides indices across 8 dimensions: limitations in physical activities (PF), limitations in social activities (SF), limitations in usual physical role activities (RP), limitations in usual role activities because of emotional problems (RE), bodily pain (BP), general mental health (psychological distress and well-being; MH), vitality (energy and fatigue; VT) and general health perception (GH). It also provides 2 summary scales; the physical and mental component summary scales (PCS and MCS). Higher scores reflect better health status (22).
Neck Disability Index (NDI). Severity of disability was measured using the NDI. The NDI consists of 10 items addressing function and activities of: personal care, lifting, reading, work, driving, sleeping and recreational activities as well as pain intensity, concentration and headache. (23). There are 6 response alternatives for each item, ranging from no disability (0) to total disability (10). A higher score indicates more pain and disability.
Self-Efficacy Scale. A 20-item Functional Self-Efficacy Scale (24) was used to measure the patients’ expectations of their own capability to accomplish certain tasks and activities of daily living. This version of the self-efficacy scale was originally developed for chronic back pain patients (24), but has been used and tested also for neck-pain conditions (25). Higher scores indicate a higher functional self-efficacy.
Tampa Scale of Kinesiophobia. Fear of re-injury due to movement was assessed using the Tampa Scale of Kinesiophobia (TSK) (26). The TSK is a 17-item questionnaire where each item is rated on a 4-grade Likert scale with scoring alternatives ranging from “strongly agree” to “strongly disagree”. A higher score indicates more kinesiophobia.
Disability of the Arm, Shoulder and Hand. The validated Swedish version (27) of the Disability of the Arm, Shoulder and Hand (DASH) questionnaire was used to measure upper extremity disability and symptoms (28). It consists of 30-items where each item is scored on a 1- to 5-point scale, ranging from “no difficulty” or “no symptom” to “unable to perform activity” or “very severe symptom”. A higher score means more disability.
Additional questions. Aspects that we considered to be of importance, but not covered by the other questionnaires, were addressed by complementary questions regarding symptoms, body functions and activity limitations (see Table II). A 6-level scale was used for each question with alternatives corresponding to: (1) not at all/nothing, (2) weak/mildly, (3) moderate, (4) quite high/somewhat strong, (5) high/strong, (6) almost unbearable/maximal.
| Table II. Variables selected for multivariate (PLS) regression of pointing acuity |
| Total scores/ Index scores | From NDI | From DASH | Additional questions |
| TSK | Pain intensity | Difficulty opening a tight or new jar | Symptom duration |
| Self-Efficacy Scale | Headache | Difficulty placing an object on a shelf above your head | VAS |
| SF-36 PF | Concentration difficulties | Difficulty doing heavy household chores | Difficulties with lifting |
| SF-36 BP | Sleeping disturbance | Difficulty carrying a shopping bag or briefcase | Difficulties with carrying |
| SF-36 GH | | Difficulty carrying a heavy object | Difficulties with throwing |
| SF-36 VT | | Difficulty changing a light bulb overhead | Difficulty taking a shirt off and on |
| SF-36 SF | | Weakness in the arms, shoulder or hand | Difficulty bending the head forward |
| SF-36 MH | | Paraesthesia in arms, shoulder or hands | Difficulty bending the head backward |
| | | Pain in the arm, shoulder or hand | Difficulty bending the head to the right |
| | | | Difficulty bending the head to the left |
| | | | Difficulty turning the head to the right |
| | | | Difficulty turning the head to the left |
| | | | Dizziness |
| | | | Balance disturbance |
| | | | Sensory disturbance |
| | | | Clumsiness of the hands |
| | | | Tenderness in the neck |
| | | | Neck pain during rest |
| | | | Neck pain during activity |
| VAS: visual analogue scale; NDI: Neck Disability Index; DASH: Disability of the Arm, Shoulder and Hand; TSK: TAMPA Scale of Kinesiophobia; PLS: partial least squares; SF-36 PF: short form-36 physical functioning; SF-36 BP: short form-36 bodily pain; SF-36 GH: short form-36 general health; SF-36 VT: short form-36 vitality; SF-36 SF: short form-36 social functioning; SF-36 MH: short form-36 mental health. |
Statistics
Statistics were calculated using SPSS for Windows 13.0 or SIMCA-P 11.0 (for PLS-analyses only, see below) and p-values lower than 0.05 were considered significant.
First, to test for possible group differences in the dependent measures between groups, VE along each coordinate axis (X, Y and Z) was analysed with mixed model analysis of variance (ANOVA) and Peak velocity and Movement time with univariate ANOVA.
As pointed out in the introduction, comparisons to identify differences in end-point acuity between groups in full-vision conditions may, due to Fitts’ Law, be of questionable worth in the absence of control for movement speed. Therefore, Peak velocity was used as covariate in a mixed-model analysis of covariance (ANCOVA) with Coordinate axis (X-, Y- and Z) as within-subject factors and Group (NS, WAD and CON) as between-subject factor. Thereafter, univariate ANCOVAs were performed for specific comparison of pre-planned contrasts (equivalent to Dunnett’s t two-sided post-hoc t-test) between neck-pain subjects and controls for the different coordinate axes.
Multivariate regression analysis
To explore the pattern of different symptoms and levels of functioning in the neck-pain subjects and associations to end-point acuity, we used PLS projection to latent structures (29), a regression extension of principal component analysis (PCA). While PCA detects latent structures of variables in one block (X), PLS methods can be used to reveal latent relationships between 2 blocks of variables (X and Y). The motivation for using PLS instead of traditional multivariate methods (i.e. multiple linear regression) reside in the technique’s ability to analyse many non-independent (i.e. collinear) variables. Other advantages are that PLS can handle noisy data structures, fewer observations than predictor variables and missing data (14). Moreover, PLS allows exploration of underlying relations and trends even when the functional form governing the relationship between predictors and response is not fully known. Here we used Orthogonal PLS (O-PLS) (30), which separates the variance in X that is correlated to Y from the variance in X that is uncorrelated (orthogonal) to the Y-variable. This is useful when there is a large amount of (Y-) unrelated variation in the data-set and makes the interpretation of the model more straightforward and accurate than normal PLS analysis, since O-PLS, in the case of a single Y, gathers the predictive X-variation in the first component (30).
PLS models, like multiple linear regression models, are described with the statistical parameters explained variation (R2) and predicted variation (Q2). Explained variation in PLS applies to both response (R2Y) as well as predictors (R2X) and Q2 is a cross-validation parameter calculated to test the validity of the model against over-fitting. The relative contribution of each x-variable to the PLS model (i.e. the correlation to Y and to the projections in X-space) is expressed as a VIP-value (variable importance in the projection): VIP larger than 1.0 is considered as influential (significant) while values lower than 0.5 indicate unimportant variables. The interval between 0.5 and 1.0 is a “grey zone” where the importance level depends on the size of the data-set (14). We considered VIP-values larger than 1 and with a confidence interval not including 0.5 to indicate a significant x-variable for a model.
To control for the effect of between-subjects variability in movement speed on end-point variability, we used the residuals from a linear regression model (VE as dependent variable and Peak velocity as predictor) as the response variable (Y) in the PLS analysis. These residuals (VEr) were calculated separately for each coordinate axes, thus, representing a velocity controlled VE in each direction. As predictors (X-variables) we used the subscales PF, SF, BP, MH, VT and GH from the SF-36 and the total scores of the TSK and SES, since each of these subscales/scores may be considered distinct theoretical concepts. The total scores for the NDI and DASH were not used since they are constructed from items representing several different theoretical concepts. Instead, the individual questions were used, excluding the ones which we considered not having any reasonable association to pointing acuity, unless univariate correlation analysis indicated such an association. The additional questions on symptom and physical functioning and the VAS pain ratings were also included. In total, 40 variables (Table II) were entered as predictors into the models. The analyses were performed on mean-centred and scaled data (14) and data distributions for all variables were evaluated and transformed (e.g. log transformed) if recommended by the built in function of the software (SIMCA-P 11.0). If the variance was found to be negligible, the variable was removed from the model. Outliers were identified as clearly deviating observations on score and residual plots (14).
RESULTS
End-point variability
Descriptive data for VE, Peak velocity and Movement time are given in Table III. The effect of Group on VE was found to be non-significant (mixed-model ANOVA: F(2,64) = 1.823, p = 0.170) for both neck-pain groups (NS-CON, p = 0.170; WAD-CON, p = 0.358, Dunnett’s t two-sided post-hoc t-test). Likewise was the effect of Group on Peak velocity non-significant (univariate ANOVA: F(1,64) = 2.354, p = 0.103), although there was a trend for lower Peak velocity in the WAD group (Dunnett’s t two-sided post-hoc t-test; NS-CON p = 0.456; WAD-CON p = 0.062). For the variable Movement time there was a significant difference between WAD and CON (F(1,64) = 3.18, p = 0.048, Dunnett’s t two-sided post-hoc t-test; NS-CON p = 0.534; WAD-CON p = 0.028).
| Table III. Mean with standard deviation within parentheses of Variable Error (VE) (cm) along the horizontal- (X), depth- (Y) and vertical axis (Z), peak velocity (cm/s) and movement time (s) |
| Group | VE-x (horizontal) | VE-y (depth) | VE-z (vertical) | Peak velocity | Movement time |
| NS | 0.34 (0.27) | 0.35 (0.17) | 0.27 (0.10) | 283 (74) | 0.88 (0.20) |
| WAD | 0.29 (0.14) | 0.35 (0.18) | 0.24 (0.10) | 257 (64) | 0.99 (0.30)* |
| CON | 0.26 (0.08) | 0.26 (0.09) | 0.22 (0.06) | 307 (87) | 0.82 (0.20) |
| *p < 0.05 (Dunnett’s t two-sided post-hoc t-test with the control group). CON: control, NS: non-specific and WAD: whiplash-associated disorders. |
In order to account for the speed–accuracy trade-off, we used Peak velocity as covariate in a mixed-model ANCOVA of VE with Group as a between-subject factor and Coordinate axis as within-subject factor. The analysis revealed significant main effects of Group (F(2,63) = 4.324, p = 0.017), Coordinate axis (F(2,126) = 3.359, p = 0.038) as well as for the covariate Peak velocity (F(1,63) = 17.820, p < 0.001). However, the interaction effect Coordinate axis × Group was non-significant (F(4,126) = 1.267, p = 0.286), indicating differences in VE between the 3 coordinate axes but a similar pattern within the groups. Thus, the results confirmed that peak velocity was a strong modifier of VE. The subsequent pair-wise comparisons between groups revealed differences between CON–NS (Dunnett’s t two-sided post-hoc t-test; p = 0.020) and CON–WAD (p = 0.034). Post-hoc comparisons of the 3 coordinate axes showed that VE in the Z-direction was significantly lower compared with both X- and Y-direction (Tukey’s p = 0.002 and p < 0.001, respectively), while X compared with Y did not differ (p = 0.127).
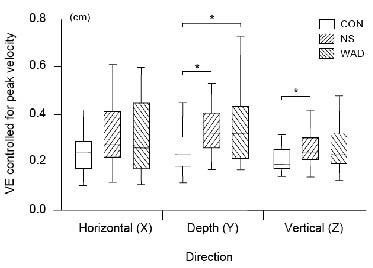
Although the interaction Coordinate axis × Group was non-significant we chose to test possible group differences in VE along the 3 coordinate axes with univariate ANCOVAs. Descriptive statistics of the data and significant group differences are shown in Fig. 2. For NS compared with CON, significant differences were found in both depth (Y-axis) and vertical (Z-axis) direction (Dunnett’s t two-sided post-hoc t-test; p = 0.030 and p = 0.032, respectively) while differences in the horizontal direction (X-axis) failed to reach significance (p = 0.086). WAD compared with CON differed in depth direction (VE-Y; p = 0.010) but not along the horizontal and vertical axes (p = 0.200 and p = 0.164, respectively).
Fig. 2. Box-plots (interquartile range) for the end-point variable error (VE) in cm controlled for peak velocity for the 3 groups (control (CON), non-specific (NS) and whiplash-associated disorders (WAD)) and error directions (horizontal, depth and vertical). Asterisks indicate significant differences of p < s0.05 (Dunnett’s t two-sided post-hoc with the CON group).
Associations between end-point variability and self-rated characteristics
Since the ANCOVA for VE revealed significant group differences we studied associations between self-rated characteristics and end-point acuity controlled for movement speed (VEr; see Methods) in the neck-pain groups using PLS regression. The analysis was performed separately for the 2 neck-pain groups using the self-ratings as predictors and the VEr in depth direction as response variable. We chose VEr in depth direction as response variable since the variability in depth was largest and discriminated both neck-pain groups from the controls, and because of the fact that VEr in all 3 directions were correlated (data not shown). Tables IV and V show the significant predictor variables from the PLS models.
| Table IV. Orthogonal Partial Least Squares analysis of Variable Error for the non-specific group using self-assessed patient characteristics as predictors. The Variable Influence on Projection (VIP), the lower limit of the confidence interval (CI) for the VIP and Pearson’s correlation coefficients (r) are shown for predictors with VIP > 1 and a lower limit of VIP CI > 0.5 |
| Predictor | VIP | CI | r |
| Neck rotation left | 2.18 | 1.54 | 0.59* |
| Neck rotation right | 2.16 | 1.52 | 0.58* |
| Neck lateral flexion left | 1.90 | 1.51 | 0.51* |
| Neck lateral flexion right | 1.90 | 1.53 | 0.51* |
| Neck flexion | 1.80 | 0.75 | 0.49* |
| Take a shirt off and on | 1.65 | 0.94 | 0.44* |
| Neck extension | 1.60 | 1.01 | 0.44* |
| *p < 0.05. |
| Table V. Orthogonal Partial Least Squares analysis of Variable Error for the whiplash-associated disorders group using self-assessed subject characteristics as predictors. The Variable Influence on Projection (VIP), the lower limit of the confidence interval (CI) for the VIP and Pearson’s correlation coefficients (r) are shown for predictors with VIP > 1 and a lower limit of VIP CI > 0.5 |
| Predictor | VIP | CI | r |
| Bodily Pain (SF-36) | 1.74 | 1.40 | –0.67* |
| Balance | 1.61 | 0.62 | 0.62* |
| Social Functioning (SF-36) | 1.52 | 1.01 | –0.54* |
| VAS | 1.32 | 0.65 | 0.49* |
| Neck extension | 1.28 | 0.71 | 0.50* |
| Carry a shopping bag (DASH) | 1.22 | 0.83 | 0.47* |
| Lifting | 1.16 | 0.50 | 0.45* |
| Carrying | 1.16 | 0.54 | 0.45* |
| Neck lateral flexion left | 1.07 | 0.50 | 0.41 |
| *p < 0.05. VAS: visual analogue scale; DASH: Disability of the Arm, Shoulder and Hand; SF-36: Short Form 36. |
For the NS group the PLS model explained 68.3% of the variance in VEr (R2Y = 0.683, R2X = 0.122, Q2 = 0.398). One NS subject was excluded from the model due to a serious “observation risk” detected on residual plots (i.e. the subject was far from the centre in X-space and had a major deviating Y-residual when the subject was included compared with when it was excluded from the model). The model revealed 7 significant predictors (Table IV), 6 representing problems in performing neck movements and one related to problems in dressing situations (putting on and taking off a shirt). The PLS-model for the WAD group explained 42.6% of the variation in VEr (R2Y = 0.426, R2X = 0.387, Q2 = 0.249). The model revealed 9 significant predictors (Table V). Similar to the NS group, end-point variability was associated with problems in performing neck movements, but, in addition, also with variables representing pain as well as limitations in activities involving lifting/carrying, poor balance, and social functioning.
Discussion
Summary of results
This study demonstrates that people with chronic neck pain of traumatic as well as non-traumatic aetiology can have reduced end-point acuity in goal-directed reaching. The acuity reduction was most pronounced in the depth direction, although not statistically different from the reduction in the horizontal and vertical directions. In addition, we found, for both patient groups, distinct associations between end-point acuity and self-rated functioning – primarily limitations of neck movements but also of activity limitations, balance and pain.
Pointing acuity
The primary aim of the study was to test the hypothesis that subjects with chronic neck pain have higher end-point variability than healthy controls when performing fast pointing movement in 3-dimensional space under a full-vision condition, when taking the speed-accuracy trade-off into account. The ANOVA for VE showed no significant group differences. However, when controlling for movement speed (Peak velocity), we found higher end-point VE in both the neck-pain groups compared with the controls. The fact that group differences were found neither for VE (when not controlling for Peak velocity) nor Peak velocity, while differences emerged when controlling VE for Peak velocity, can be interpreted as a result of a combination of the speed-accuracy trade-off and between-subject variability in how the task was performed. That is, between-subject variability in Peak velocity (in all groups) increased the between-subject variability in VE, due to the close link between these 2 variables (e.g. 12). Eliminating this effect by controlling VE for Peak velocity revealed the acuity reduction. Hence, the results lend support to hypothesis 1 and show that the speed-accuracy trade-off should be taken into account to reveal deficits in the control of reaching acuity.
Our second hypothesis predicted that the most pronounced difference in end-point accuracy should appear in the near-far (depth) direction. This hypothesis was based on the suggestions that acuity in the near-far direction predominantly depends on proprioceptive information (9, 13), and the finding of reduced upper extremity proprioception in people with chronic neck pain (8). However, our results did not specifically confirm this prediction. Although the WAD group, as hypothesized, showed significantly higher VE than controls in depth direction only, this difference was not statistically different from the VE-differences in the other directions. The NS group exhibited significantly higher VE both in depth- and vertical direction as well as a VE-difference approaching significance also along the horizontal axis. Therefore, the similar VE-pattern across groups, indicated by the non-significant interaction effect Coordinate axis × Group in the ANCOVA, suggests that end-point acuity in neck-pain subjects is not isolated to higher variability in depth direction.
Although the results did not support our second hypothesis, this finding does not exclude proprioception as an underlying cause of the increased reaching errors in the neck-pain groups. Thus, with respect to the direction-specific sensory efficiency hypothesis (13), it is important to note that although proprioceptive information is suggested to be more important than vision for acuity in the near-far direction, there is a substantial overlap between the use of these sources of information in all directions (13). Therefore, a potential deficit in proprioception would also affect acuity in directions other than the near-far, but to a smaller extent. Considering the relatively small sample sizes in the present study, the direction-specific effect of any proprioceptive deficit in the neck-pain groups may have been too small to detect. Future studies including perturbations of proprioception and/or vision during reaching may provide deeper insight into this topic in people with chronic neck pain.
Possible mechanisms behind reduced reaching acuity
There are several possible mechanisms, peripheral as well as central, that may account for the reduced reaching acuity in the neck-pain groups. One possibility lies in a reduced acuity of the proprioceptive information from the muscle spindle system. In animal models, profound modulations of the sensitivity of muscle-spindles in the trapezius muscle, via spinal reflexes, have been shown after close intra-muscular injections of algesic or inflammatory substances (31). Such effects can have negative impact on the information content from groups of muscle spindles (32), which may compromise proprioception (6). In the PLS analysis of our data, pain-rating was a significant predictor for high end-point variability in the WAD group but not in the NS group. This ambiguous result leaves this mechanism tentative, although it cannot be excluded since inflammatory processes are not always reflected in pain perception. Another afferent mechanism that may interfere with proprioception is related to injuries or stress on peripheral nerves anywhere along the sensory pathway (e.g. 33), which possibly could corrupt the transmission of proprioceptive afferent information, as well as affect other aspects of movement control. In our data, however, the predictor variables in the PLS analyses most likely to reflect such nerve involvement; paraesthesia and sensory loss, were not associated with reaching acuity, which argues against this mechanism as a major factor behind the reduced reaching acuity in the neck-pain groups. In this context it is relevant to note that the experienced physiotherapist conducting the test noted no adverse reactions in the subjects in relation to the test. It is also possible that sensorimotor control deficits are effects of information processing or motor planning modifications driven by higher centres by virtue of pain-related factors, such as fear, stress and attention-demanding requirements. However, the results from the PLS-analyses did not support any direct involvement of such factors since neither the TSK nor the question on concentration difficulties were significant predictors in any of the models.
A strong association was found between end-point reaching acuity and self-rated neck function for both the NS and WAD groups, implying that impairment of some aspects of neck function directly affects the control of reaching movements. This is in line with the fact that the neck plays a key role in the control of spatially oriented movements. When reaching for an object, vision provides information of the location of the object in an external coordinate system. Proprioception, on the other hand, provides information on the location of the arm and hand in an intrinsic coordinate system. Hence, the position of the head and neck is used as a reference in the integration of coordinate systems (34, 35). Together with the findings that neck pain is associated with sensorimotor dysfunctions of the neck (1), this suggests that impaired sensorimotor function of the neck may be one mechanism behind the reduced reaching acuity in the neck-pain groups. Another related explanation for the association between self-rated neck function and impaired reaching acuity could be an inability to direct vision fast enough towards the goal during the movement, since neck function can affect oculomotor control (36). In the WAD group, a strong association was also present between poor reaching acuity and self-reported balance problems. Observations of poor balance in neck-pain conditions are frequent (e.g. 3, 37) and the role of the neck for postural control is well known (for references see (36)). Furthermore, Karlberg et al. (36) showed that restricting neck mobility using a cervical collar impairs postural control in healthy subjects, a finding that underscores the importance of neck function for balance and spatial orientation. With these studies in mind, our results indicate that balance problems and poor reaching acuity may co-exist since they can both be related to impaired neck function. Surprisingly, none of the self-rated functions that could be affected by decreased acuity of precise arm-hand movements (e.g. clumsiness in the hands or changing a light bulb) were significant predictors in the PLS models. However, such deficits can often be compensated for by performing movements slower and may therefore remain unrecognized by the individual, and thus not reflected in the self-ratings.
The findings from the PLS analyses raise several questions regarding the mechanisms behind the associations. For example, the mechanisms behind the association between pointing acuity and self-rated neck function could be explored by recording head movements during the test as well as assessing oculomotor functions such as smooth pursuit eye movements (36). Additionally, the importance of visual information vs shoulder-arm proprioception could be elucidated by manipulating available sensory input during the task (13).
Clinical relevance
Reduced acuity of goal-directed arm movements, such as pointing or reaching, can have implications for an individual’s everyday functioning, since many everyday activities depend on good precision and timing. Even seemingly small increases in end-point variability would obviously risk compromising the performance in activities such as performing sports or playing a musical instrument, as well as in many working life situations and common everyday tasks. Furthermore, in real-life tasks requiring a certain level of end-point acuity, it is conceivable that deficiencies in control of end-point acuity can be compensated for by performing movements slower. Also, a well-known strategy to cope with speed-accuracy demands is by increasing muscle co-contraction (e.g. 38). Hence, even if reduced end-point acuity can be compensated for, it can have negative consequences in terms of extra muscle tension and aggravation of muscle fatigue.
For clinical practice, the results support training regimes aimed at movement control and body awareness with special focus on the neck and upper extremity. Since, as discussed above, neck function may constitute a key function for the integration of external (vision) and internal (proprioceptive) reference frames (34, 35), training regimes that target such integration should be considered in the rehabilitation of people with neck pain.
Acknowledgements
The authors would like to thank Nisse Larson for excellent engineering support and Maria Frykman for capable administrative work. The study was funded by Alfta Research Foundation and by grants from the Swedish Governmental Agency for Innovation Systems, VINNOVA (project number 510240).
References
1. O’Leary S, Falla D, Jull G. Recent advances in therapeutic exercise for the neck: implications for patients with head and neck pain. Aust Endod J 2003; 29: 138–142.
2. Karlberg M, Persson L, Magnusson M. Reduced postural control in patients with chronic cervicobrachial pain syndrome. Gait Posture 1995; 3: 241–249.
3. Michaelson P, Michaelson M, Jaric S, Latash ML, Sjölander P, Djupsjöbacka M. Vertical posture and head stability in patients with chronic neck pain. J Rehabil Med 2003; 35: 229–235.
4. Kristjansson E, Dall’Alba P, Jull G. A study of five cervicocephalic relocation tests in three different subject groups. Clin Rehabil 2003; 17: 768–774.
5. Revel M, Andre-Deshays C, Minguet M. Cervicocephalic kinesthetic sensibility in patients with cervical pain. Arch Phys Med Rehabil 1991; 72: 288–291.
6. Johansson H, Arendt-Nilsson L, Bergenheim M, Blair S, van Dieen J, Djupsjöbacka M, et al. Epilogue: an integrated model for chronic work-related myalgia “Brussels Model”. In: Johansson H, Windhorst U, Djupsjöbacka M, Passatore M, editors. Gävle: Gävle University Press; 2003, p. 291–300.
7. Knox JJ, Beilstein DJ, Charles SD, Aarseth GA, Rayar S, Treleaven J, et al. Changes in head and neck position have a greater effect on elbow joint position sense in people with whiplash-associated disorders. Clin J Pain 2006; 22: 512–518.
8. Sandlund J, Djupsjöbacka M, Ryhed B, Hamberg J, Bjorklund M. Predictive and discriminative value of shoulder proprioception tests for patients with whiplash-associated disorders. J Rehabil Med 2006; 38: 44–49.
9. van Beers RJ, Wolpert DM, Haggard P. When feeling is more important than seeing in sensorimotor adaptation. Curr Biol 2002; 12: 834–837.
10. Woodworth RS. The accuracy of voluntary movement. Psychol Rev 1899; 3 Suppl 3: 1–119.
11. Fitts PM. The information capacity of the human motor system in controlling the amplitude of movement. J Exp Psychol 1954; 47: 381–391.
12. Elliott D, Helsen WF, Chua R. A century later: Woodworth’s (1899) two-component model of goal-directed aiming. PsyB 2001; 127: 342–357.
13. van Beers RJ, Sittig AC, Gon JJ. Integration of proprioceptive and visual position-information: an experimentally supported model. J Neurophysiol 1999; 81: 1355–1364.
14. Eriksson L, Johansson E, Kettaneh-Wold N, Wold S. Introduction to multi- and megavariate data analysis using the projection methods (PCA & PLS). Umeå: Umetrics AB; 1999.
15. Wold S, Sjostrom M, Eriksson L. PLS-regression: a basic tool of chemometrics. Chemometrics and Intelligent Laboratory Systems 2001; 58: 109–130.
16. Margolis RB, Chibnall JT, Tait RC. Test-retest reliability of the pain drawing instrument. Pain 1988; 33: 49–51.
17. McCarthy CJ, Cairns MC. Why is the recent research regarding non-specific pain so non-specific? Man Ther 2005; 10: 239–241.
18. Maravita A, Iriki A. Tools for the body (schema). Trends Cognitive Sci 2004; 8: 79–86.
19. Domkin D, Laczko J, Djupsjöbacka M, Jaric S, Latash ML. Joint angle variability in 3D bimanual pointing: uncontrolled manifold analysis. Exp Brain Res 2005; May;163:44-57.
20. van Beers RJ, Sittig AC, Denier van der Gon JJ. How humans combine simultaneous proprioceptive and visual position information. Exp Brain Res 1996; 111: 253–261.
21. Huskisson EC. Measurement of pain. Lancet 1974; 2: 1127–1131.
22. Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992; 30: 473–483.
23. Vernon H, Mior S. The Neck Disability Index: a study of reliability and validity. J Manipulative Physiol Ther 1991; 14: 409–415.
24. Altmaier E, Russel D, Kau C, Weinstein J. Role of self-efficacy in rehabilitation outcome among chronic low back pain patients. J Couns Psychol 1993; 40: 335–339.
25. Denison E, Asenlof P, Lindberg P. Self-efficacy, fear avoidance, and pain intensity as predictors of disability in subacute and chronic musculoskeletal pain patients in primary health care. Pain 2004; 111: 245–252.
26. Kori S, Miller R, Todd D. Kinesophobia: a new view of chronic pain behaviour. Pain Management 1990; Jan/Feb: 35–43.
27. Atroshi I, Gummesson C, Andersson B, Dahlgren E, Johansson A. The disabilities of the arm, shoulder and hand (DASH) outcome questionnaire: reliability and validity of the Swedish version evaluated in 176 patients. Acta Orthop Scand 2000; 6: 613–618.
28. Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) (corrected). The Upper Extremity Collaborative Group (UECG). Am J Ind Med 1996; 29: 602–608.
29. Wold S, Johansson E, Cocchi M. PLS : Partial Least Squares Projections to Latent Structures. In: Kubinyi H, editor. 3D QSAR in Drug DESIGN: theory, methods and applications. Leiden: ESCOM Science Publishers; 1993, p. 523–550.
30. Trygg J, Wold S. Orthogonal projections to latent structures (O-PLS). J Chemometrics 2002; 16: 119–128.
31. Wenngren BI, Pedersen J, Sjölander P, Bergenheim M, Johansson H. Bradykinin and muscle stretch alter contralateral cat neck muscle spindle output. Neurosci Res 1998; 32: 119–129.
32. Pedersen J, Ljubisavljevic M, Bergenheim M, Johansson H. Alterations in information transmission in ensembles of primary muscle spindle afferents after muscle fatigue in heteronymous muscle. Neuroscience 1998; 84: 953–959.
33. Greening J, Lynn B, Leary R. Sensory and autonomic function in the hands of patients with non-specific arm pain (NSAP) and asymptomatic office workers. Pain 2003; 104: 275–281.
34. Berger M, Lechner-Steinleitner S, Kozlovskaya I, Holzmuller G, Mescheriakov S, Sokolov A, et al. The effect of head-to-trunk position on the direction of arm movements before, during, and after space flight. J Vestib Res 1998; 8: 341–354.
35. Fookson O, Smetanin B, Berkinblit M, Adamovich S, Feldman G, Poizner H. Azimuth errors in pointing to remembered targets under extreme head rotations. Neuroreport 1994; 5: 885–888.
36. Karlberg M, Magnusson M, Johansson R. Effects of restrained cervical mobility on voluntary eye movements and postural control. Acta Otolaryngol 1991; 111: 664–670.
37. Field S, Treleaven J, Jull G. Standing balance: A comparison between idiopathic and whiplash-induced neck pain. Man Ther 2007; February 14 [Epub ahead of print].
38. Meulenbroek RGJ, Van Galen GP, Hulstijn M, Hulstijn W, Bloemsaat G. Muscular co-contraction covaries with task load to control the flow of motion in fine motor tasks. Biol Psychol 2005; 68: 331–352.