OBJECTIVE: Patients in intensive care exhibit a high degree of loss of muscle mass. Appropriate instruments are needed to document muscle wasting in these patients. The aim of this pilot study was to describe muscle wasting in patients in the intensive care unit.
DESIGN: Two-fold study setting: prospective longitudinal and cross-sectional single-blind.
Patients: A total of 118 patients in the intensive care unit (length of stay 1–98 days; male:female ratio 88:30; age 55 ± 17 years) were included in a two-fold study setting.
METHODS: Muscle layer thickness of the M. quadriceps femoris was documented using ultrasound measurement at well-defined points. Seventeen pilot-patients were measured twice; at baseline and after 28 days. In another group of 101 patients, muscle layer thickness was determined once after a random length of stay. The results of both groups were compared and correlated.
RESULTS: In both groups, M. quadriceps femoris thickness showed a significant negative correlation with length of stay in the intensive care unit (p < 0.01). Furthermore, muscle wasting in intensive care patients could be described using a logarithmic function.
CONCLUSION: Loss of muscle mass shows a negative correlation with length of stay, and seems to be higher during the first 2–3 weeks of immobilization/intensive care unit stay.
Ultrasound is a valid and practical measurement tool for documenting muscle mass (e.g. muscle layer thickness) as part of the daily routine at an intensive care unit.
Key words: muscle wasting, intensive care, diagnostic ultrasound, muscle layer thickness.
J Rehabil Med 2008; 40: 185–189
Correspondence address: Richard Crevenna, University Department of Physical Medicine and Rehabilitation, Medical University of Vienna/MUW, Währingergürtel 18-20, AT-1090, Vienna, Austria. E-mail: richard.crevenna@meduniwien.ac.at
Submitted December 31, 2006; accepted September 20, 2007.
Introduction
Critically ill patients exhibit a dramatic loss of lean body mass, particularly skeletal muscle, irrespective of the adequacy of nutritional support (1–4).
Muscle wasting is one of many common problems patients in intensive care unit (ICU) have to deal with. These problems have been specified in detail using the International Classification of Functioning, Disability and Health (ICF) (5–9).
Skeletal muscle wasting in the critically ill is often masked by fluid retention. In these circumstances the normal anthropometric methods of assessing changes in body mass and composition are not applicable, as the techniques all assume a normal state of hydration (10). Until recently, the only methods that reliably measure wasting of lean tissue in critically ill patients with presence of severe fluid retention have been in vitro neutron activation analysis (IVNAA) (11) or assessing changes in muscle fibre area using repeated muscle biopsies (12–15). The former involves radiation and is not generally available, and the latter is invasive and time-consuming. Both methods can only really be used in research settings. Therefore, a simple, non-invasive, clinically applicable method is needed to assess rates of muscle wasting during critical illness in the presence of severe oedema.
Strength measurement is not feasible at the intensive care unit (ICU) for documenting muscle wasting, but there is a proven relationship between strength and muscle thickness of the human quadriceps femoris muscle (16, 17). To evaluate the physiological muscle morphology of human thigh and leg muscles, several similar imaging techniques, such as computerized tomography (CT), magnetic resonance imaging (MRI) or ultrasound, can be employed (18–21). Muscle wasting has been observed by means of ultrasound after bed rest, immobilization after operation, and in adults with severe cerebral palsy (22–27).
Muscle wasting in patients in the ICU is a recent field of research and, as yet, there is a lack of published data (28–30). To our knowledge, there is no published clinical research documenting changes in muscle mass and morphology in patients in the ICU during immobilization over a median time-period of 7 days.
The purpose of the present study was: (i) to measure muscle wasting in patients in the ICU over a period of 28 days; (ii) to determine whether a relationship could be identified between a representative population of patients in the ICU and their length of stay (LOS) at an ICU; and (iii) to verify whether ultrasound measurements are a valid and practical measurement tool to document muscle mass (e.g. muscle layer thickness (MLT)) in daily routine in these patients.
Methods
All the procedures described in this study were approved by the local ethics committee (number 037/2004) and informed consent was obtained from all patients or proxy. The study was conducted at the ICU – Department of Anesthesiology and Intensive Care, General Hospital of Vienna, Medical University of Vienna, Austria.
Study design
It is difficult to establish a study in an ICU to observe patients over several weeks due to the high turn-over and morbidity of these patients. Therefore, a “two-fold” study setting (group A, B) was chosen to document muscle wasting (e.g. loss of MLT) over several weeks, avoiding any selection bias.
In a prospective longitudinal setting (group A) muscle wasting of pilot patients (n = 17) was documented: (i) at “baseline” (starting with the next day after their referral to the ICU); and (ii) after 28 days.
In a cross-sectional setting (group B), muscle wasting of a larger group of patients (n = 101) was determined only once, but after different time periods (after their referral to the ICU) to describe MLT at variable lengths of stay.
To our knowledge there are no published data concerning the effect size of muscle wasting in patients treated for a long time in the ICU, which could be used for a sample size calculation.
Study population
In the year 2004, 968 patients transferred to the ICU of the general hospital of Vienna had a LOS longer than 7 days and survived their critical diseases (dismissed and deducted patients in 2004, annual report entire General Hospital of Vienna).
A total of 125 patients (88 men and 30 women, aged 55 ± 17 years) on a standard drug treatment were included in a two-fold study setting during 2004. A total of 118 patients completed the study (Table I).
Exclusion criteria were: a stay at the ICU shorter than 28 days for group A and shorter than 7 days for group B; age less than 19 years; neuromuscular disorders or critical illness neuropathy; and patients with cancer treated with a palliative intention.
| Table I. Baseline characteristics of patients in intensive care units in group A and B. |
| Group | A | B |
| Sex (m/f) | 14/3 | 74/27 |
| Age (years), mean (SD) | 55 (17) | 55 (15) |
| Height (m), mean (SD) | 1.74 (0.12) | 1.76 (0.9) |
| Weight (kg), mean SD) | 84 (21) | 81 (17) |
| Body surface (m²), mean (SD) | 2.02 (0.3) | 2 (0.25) |
| Main diagnosis | | |
| Polytrauma | 5 | 29 |
| Cardiovascular | 4 | 23 |
| Transplantation | 2 | 8 |
| Pneumonia | 3 | 12 |
| Cancer | 2 | 14 |
| Abdominal disorders | 1 | 9 |
| Wound infection | – | 4 |
| Other diseases | – | 2 |
| Incomplete (death/transfer) | 5/2 | – |
| SD: standard deviation. |
Outcome measurements
MLT of the M. vastus intermedius and M. rectus femoris were documented using high-resolution ultrasound. In group A, MLT of 17 patients was measured twice, at baseline (after a random LOS) and after 28 days. In group B, MLT of 101 patients was determined only once (after a random LOS). The results of both groups were compared and correlated with demographic and clinical data.
To detect muscle wasting, MLT of the quadriceps femoris muscle (M. vastus intermedius and M. rectus femoris) was measured by high-resolution real-time ultrasonography at well-defined points, as described in other studies (22, 24, 25). MLT of the M. vastus intermedius and M. rectus femoris was assessed bilaterally: (i) at the border between the lower and upper two-thirds, and (ii) at the mid-point between the anterior superior iliac spine and the upper pole of the patella, with the patient in a supine position and the legs relaxed lying flat in extension. Mean MLT was calculated by mean of the 2 measurements (measuring points (i) and (ii)) on each leg. The coefficient of variation for a single MLT measurement is close to the 3% obtained in other studies (23, 30). MLT measurement used in the present study improves the coefficient to 1.3% (23).
All ultrasound examinations were performed by the same operator, who was blinded to the data analysis, with a portable ATL ultrasound system (HDI-1000, ATL©, Bothell, USA), using a L7-4 transducer with a 5-cm linear array footprint.
For supplementation of the MLT measurements, the circumference of the thigh was assessed with a tape measure bilaterally between the 2 previously-defined ultrasound measuring points.
Statistics
Data from all patients were analysed with the Statistical Package for Social Sciences (SPSS 13 for Windows). Descriptive statistics were calculated and presented as mean (± standard error of the mean (SEM)) as appropriate. A correlation matrix was generated to assess the degree of correlation among the variables (sex, age, height, weight, body surface, thigh circumference, MLT, MLT difference after 28 days at the ICU (MLTD), LOS). Pearson’s product-moment coefficient of correlation was used for parametric data. Stepwise multiple regression analysis was performed to identify dependent variables on MLT. Curve estimation for best-fit was used to describe MLTD with respect to LOS at the ICU. Null hypothesis was rejected when p < 0.05.
Results
Group A
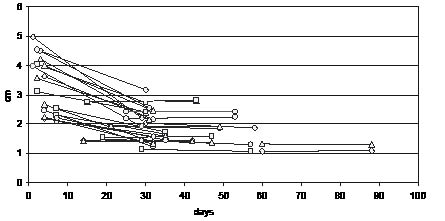
In 17 patients, ultrasound representations of MLT in both legs separately were characterized by a decrease in 27 thighs and by an increase in 7 thighs (Fig. 1).
Fig. 1. Muscle layer thickness changes in group A, from the first (after a random length of stay) to the second measurement (after 28 days) in both thighs.
Correlation analysis showed a high significant negative correlation between MLT of the right (p = 0.005) and left thigh (p = 0.004), MLTD for the right (p = 0.006) and left thigh (p = 0.003) and LOS at the ICU at baseline measurement. Height revealed a high significant negative correlation (p = 0.003), and age a significant positive correlation (p = 0.011) with LOS. Thigh circumference, sex, weight and body surface did not correlate with LOS at the ICU at baseline measurement.
Stepwise multiple regression analysis showed that LOS at the ICU at baseline measurement was the only variable with an influence on MLTD on the right (p = 0.006) and left thigh (p = 0.003).
Group B
In 101 patients the correlation analysis showed a high significant negative correlation between MLT of the right (p < 0.0001) and left thigh (p < 0.0001) and LOS at the ICU at baseline measurement. Thigh circumference of both legs revealed a high significant negative correlation (p = 0.003) with LOS. Age showed a high significant positive correlation (p = 0.001) with LOS. Height revealed a significant negative correlation (p = 0.031) with LOS. Sex, weight and body surface did not correlate with LOS at the ICU at baseline measurement.
Stepwise multiple regression analysis showed that LOS at the ICU at baseline measurement (p < 0.0001) and thigh circumference (p = 0.006) were the only variables that influenced MLT on the right thigh.
Influences on MLT on the left thigh were LOS at the ICU at baseline measurement (p < 0.0001), thigh circumference (p < 0.0001) and height (p = 0.016).
Additional calculations (multiple regression analyses) to study the effects of height on LOS while controlling the age effect were performed. No effects were found.
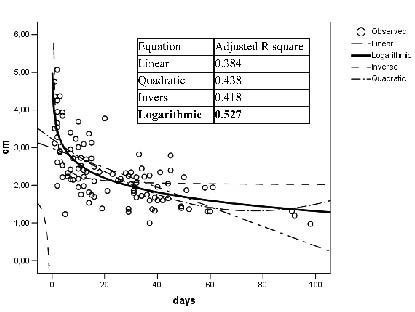
Curve estimation for best fit revealed that the logarithmic function fits best to MLT of both thighs with respect to LOS at the ICU (Fig. 2).
Fig. 2. Example of curve estimation: muscle layer thickness of the right thigh in group B with respect to the length of stay at the intensive care unit at baseline measurement.
Discussion
To summarize, muscle mass losses of the M. vastus intermedius and M. rectus femoris show a correlation with LOS at the ICU. Muscle mass losses during the first 2–3 weeks of stay in the ICU are considerably higher than in all other patient collectives examined. There are differences concerning speed of muscle wasting in the patients during their stay. Muscle wasting can be described best by using a logarithmic function. MLT measuring is more suitable for muscle atrophy documentation than size quantification with a measuring tape.
This study aimed to determine whether real-time ultrasound could provide quantitative data that: (i) elucidates muscle wasting in MLT of the M. vastus intermedius and M. rectus femoris of the patients over a period of 28 days; and (ii) determines whether a relationship can be identified between a representative population of patients and their LOS at an ICU.
Establishing a study setting on an ICU observing patients over several weeks is difficult because of the high turn-over and morbidity of these patients. To solve this problem, a two-fold study setting was chosen to limit drop-out rates, to be able to document muscle wasting over several weeks and to calculate a realistic model of a whole ICU population.
As outlined in the introduction, muscle wasting in patients in ICU is a recent field of research. To our knowledge there is only one study, which documented muscle wasting using an imaging technique in an usual ICU setting. Reid et al. (30) demonstrated an ultrasound technique for documenting muscle wasting at 3 different muscle groups.
Imaging techniques for studying and measuring muscle mass in vivo have included MRI and ultrasound (25, 31–33). Recent studies compared these imaging techniques and found no significant differences between them in measurements of muscle mass (18–20).
Examination of muscle wasting in patients in ICU with ultrasound was chosen because it is more practicable and feasible than using CT or MRI on an ICU and overcomes many of the problems associated with anthropometric and body composition measurements (10). Several measuring parameters have been described for documenting muscle mass with ultrasound (16, 25, 26). In the present study MLT of the M. quadriceps femoris was used for documenting muscle wasting because: (i) it is well described (22–27) and easy to determine even in highly reduced muscle architecture; (ii) M. quadriceps femoris is important in the remobilization process because it flexes the hip and extends the knee simultaneously (34) while standing up; and (iii) there is a proven relationship between strength and muscle thickness of the human quadriceps femoris muscle (16, 17).
The present study shows that real-time ultrasound was indeed useful in quantifying configurational changes of the M. quadriceps femoris architecture. Muscle wasting in MLT of the M. vastus intermedius and M. rectus femoris showed a high significant correlation with the LOS at the ICU in both groups A and B. Stepwise multiple regression analysis showed that LOS at the ICU was the only variable with an influence on MLTD in both groups.
As outlined before, a two-fold study setting is needed to detect muscle wasting in these patients over a period of at least 28 days, and to calculate a realistic model of a whole ICU population. Using only one group of patients, with a fixed time period of 28 days between the MLT measurements, would have led to an election bias including only patients with an expected stay of several weeks.
The exact underlying mechanisms of muscle mass losses are still unknown (2–4, 30). Studies that examined changes in body mass and composition in these patients observed a loss of lean tissue (MLT) or protein loss from skeletal muscles (2–4, 30).
In the present study, the observed muscle mass losses during the first 2–3 weeks in ICU were similar to those described by Reid et al. (30), but considerably higher than in all other patient collectives or healthy subjects in bed rest experiments (22–27, 35) examined using ultrasound. Reid et al. (30) showed that energy balance probably made no apparent difference to the rate of lean tissue loss, and implies that energy balance makes no difference to the rate of muscle wasting. Related to the presented data, the enormous rates of muscle wasting in these patients cannot be explained by bed rest at the ICU alone, and appear to be a main consequence of the disease and its treatment.
In contrast to the findings of Reid et al. (30), differences in the speed of muscle wasting in patients during their stay at an ICU were found in the presented study. Muscle mass losses in MLT of the M. vastus intermedius and M. rectus femoris can be described best by a logarithmic function. Reid et al. documented muscle wasting in patients in an ICU over a time period of between 5 and 39 (median 7) days. The observation period was much smaller than in the present study (group A: 1–60 days, mean 15 days; group B: 1–98 days, mean 24 days) and long-term effects could not be observed by Reid et al. (30).
In the present study, no correlation could be found in group A between thigh circumference measurement and LOS. In group B, thigh circumference showed a correlation with LOS at the ICU. We found 3 possible explanations: (i) muscle mass in group A was documented after a shorter LOS because of the study setting (2 measurements, at baseline and after 28 days). The patients show oedemas more often at the beginning of their stay at an ICU because of their positive water balance. (ii) Group B was a larger group of patients. (iii) Patients in group A suffered from more severe diseases then the patients in group B because of different exclusion criteria (group A: LOS ≤ 28 days; group B: LOS ≤ 7 days).
Surprisingly, height showed a significant negative correlation and age a significant positive correlation with LOS in group A and B in the present study. Furthermore, additional calculations (multiple regression analyses) to study the effects of height on LOS while-controlling the age effect did not reveal any effects of height on LOS. A possible explanation could be that younger patients are on average taller than older patients, recover faster and are earlier transferred.
Limitations of the present study include: (i) the patients received a lot of different pharmaceuticals during their stay, some of which may have effects on muscle wasting (29). Because of the mixed population of acute and chronic patients in the present study it was not possible to make a correlation between drug intake and muscle mass loss. (ii) The purpose of the present study was to describe a reliable model of muscle wasting in acute and chronic ICU patients during their stay at the ICU. It was not possible to say which patients with less MLT had less MLT before the onset of their illness and which had less MLT because they had already lost it as a result of their illness and before admission to the study. (iii) Strength measurements are not feasible at the ICU for documenting muscle wasting. The present study cannot draw any conclusions about muscle strength in the patients in ICU, although there is a proven relationship between strength and muscle thickness of the human quadriceps femoris muscle (16, 17).
In conclusion, ultrasound seems to be a valid and practical measurement tool for use in daily routine at the ICU for documenting muscle mass (e.g. MLT). Further studies are required to identify the main factors involved in muscle wasting, particularly since muscle mass loss is more distinctive in ICU patients than in other patient populations. Further research is also needed to establish rehabilitation options for patients in intensive care in order to minimize muscle wasting during their stay at the ICU.
References
1. Hart DW, Wolf SE, Herndon DN, Chinkes DL, Lal SO, Obeng MK, et al. Energy expenditure and caloric balance after burn: increased feeding leads to fat rather than lean mass accretion. Ann Surg 2002; 235: 152–161.
2. Plank LD, Hill GL. Similarity of changes in body composition in intensive care patients following severe sepsis or major blunt injury. Ann N Y Acad Sci 2000; 904: 592–602.
3. Streat SJ, Beddoe AH, Hill GL. Aggressive nutritional support does not prevent protein loss despite fat gain in septic intensive care patients. J Trauma 1987; 27: 262–266.
4. Green CJ, Campbell IT, McClelland P, Hutton JL, Ahmed MM, Helliwell TR, et al. Energy and nitrogen balance and changes in midupper-arm circumference with multiple organ failure. Nutrition 1995; 11: 739–746.
5. Grill E, Ewert T, Chatterji S, Kostanjsek N, Stucki G. ICF Core Sets development for the acute hospital and early post-acute rehabilitation facilities. Disabil Rehabil 2005; 27: 361–366.
6. Grill E, Hermes R, Swoboda W, Uzarewicz C, Kostanjsek N, Stucki G. ICF Core Set for geriatric patients in early post-acute rehabilitation facilities. Disabil Rehabil 2005; 27: 411–417.
7. Grill E, Huber EO, Stucki G, Herceg M, Fialka-Moser V, Quittan M. Identification of relevant ICF categories by patients in the acute hospital. Disabil Rehabil 2005; 27: 447–458.
8. Grill E, Quittan M, Huber EO, Boldt C, Stucki G. Identification of relevant ICF categories by health professionals in the acute hospital. Disabil Rehabil 2005; 27: 437–445.
9. Scheuringer M, Grill E, Boldt C, Mittrach R, Mullner P, Stucki G. Systematic review of measures and their concepts used in published studies focusing on rehabilitation in the acute hospital and in early post-acute rehabilitation facilities. Disabil Rehabil 2005; 27: 419–429.
10. Manning EM, Shenkin A. Nutritional assessment in the critically ill. Crit Care Clin 1995; 11: 603–634.
11. Hill GL. Implications of critical illness, injury, and sepsis on lean body mass and nutritional needs. Nutrition 1998; 14: 557–558.
12. Helliwell TR, Coakley JH, Wagenmakers AJ, Griffiths RD,
Campbell IT, Green CJ, et al. Necrotizing myopathy in critically-ill patients. J Pathol 1991; 164: 307–314.
13. Helliwell TR, Wilkinson A, Griffiths RD, McClelland P, Palmer TE, Bone JM. Muscle fibre atrophy in critically ill patients is associated with the loss of myosin filaments and the presence of lysosomal enzymes and ubiquitin. Neuropathol Appl Neurobiol 1998; 24: 507–517.
14. Griffiths RD, Palmer TE, Helliwell T, MacLennan P, MacMillan RR. Effect of passive stretching on the wasting of muscle in the critically ill. Nutrition 1995; 11: 428–432.
15. Burnham EL, Moss M, Ziegler TR. Myopathies in critical illness: characterization and nutritional aspects. J Nutr 2005; 135: 1818S–1823S.
16. Chi-Fishman G, Hicks JE, Cintas HM, Sonies BC, Gerber LH. Ultrasound imaging distinguishes between normal and weak muscle. Arch Phys Med Rehabil 2004; 85: 980–986.
17. Freilich RJ, Kirsner RL, Byrne E. Isometric strength and thickness relationships in human quadriceps muscle. Neuromuscul Disord 1995; 5: 415–422.
18. Dupont AC, Sauerbrei EE, Fenton PV, Shragge PC, Loeb GE, Richmond FJ. Real-time sonography to estimate muscle thickness: comparison with MRI and CT. J Clin Ultrasound 2001; 29: 230–236.
19. Juul-Kristensen B, Bojsen-Moller F, Holst E, Ekdahl C. Comparison of muscle sizes and moment arms of two rotator cuff muscles measured by ultrasonography and magnetic resonance imaging. Eur J Ultrasound 2000; 11: 161–173.
20. Walton JM, Roberts N, Whitehouse GH. Measurement of the quadriceps femoris muscle using magnetic resonance and ultrasound imaging. Br J Sports Med 1997; 31: 59–64.
21. Blazevich AJ, Gill ND, Zhou S. Intra- and intermuscular variation in human quadriceps femoris architecture assessed in vivo. J Anat 2006; 209: 289–310.
22. Abe T, Kawakami Y, Suzuki Y, Gunji A, Fukunaga T. Effects of 20 days bed rest on muscle morphology. J Gravit Physiol 1997; 4: S10–S14.
23. Bleakney R, Maffulli N. Ultrasound changes to intramuscular architecture of the quadriceps following intramedullary nailing. J Sports Med Phys Fitness 2002; 42: 120–125.
24. Ellis S, Kirby LC, Greenleaf JE. Lower extremity muscle thickness during 30-day 6 degrees head-down bed rest with isotonic and isokinetic exercise training. Aviat Space Environ Med 1993; 64: 1011–1015.
25. Akima H, Kawakami Y, Kubo K, Sekiguchi C, Ohshima H,
Miyamoto A, et al. Effect of short-duration spaceflight on thigh and leg muscle volume. Med Sci Sports Exerc 2000; 32: 1743–1747.
26. Uremovic M, Pasic MB, Seric V, Solter VV, Budic R, Bosnjak B, et al. Ultrasound measurement of the volume of musculus quadriceps after knee joint injury. Coll Antropol 2004; 28 Suppl 2: 227–233.
27. Ohata K, Tsuboyama T, Ichihashi N, Minami S. Measurement of muscle thickness as quantitative muscle evaluation for adults with severe cerebral palsy. Phys Ther 2006; 86: 1231–1239.
28. Klaude M, Hammarqvist F, Wemerman J. An assay of microsomal membrane-associated proteasomes demonstrates increased proteolytic activity in skeletal muscle of intensive care unit patients. Clin Nutr 2005; 24: 259–265.
29. Moukas M, Vassiliou MP, Amygdalou A, Mandragos C, Takis F, Behrakis PK. Muscular mass assessed by ultrasonography after administration of low-dose corticosteroids and muscle relaxants in critically ill hemiplegic patients. Clin Nutr 2002; 21: 297–302.
30. Reid CL, Campbell IT, Little RA. Muscle wasting and energy balance in critical illness. Clin Nutr 2004; 23: 273–280.
31. Laborde A, Rebai H, Coudeyre L, Boisgard S, Eyssette M, Coudert J.
Étude comparative de deux protocoles d’électrostimulation du quadriceps après chirurgie du ligament croisé antérieur. Étude de faisabilité. Ann Readapt Med Phys 2004; 47: 56–63.
32. Rittweger J, Frost HM, Schiessl H, Ohshima H, Alkner B, Tesch P, et al. Muscle atrophy and bone loss after 90 days’ bed rest and the effects of flywheel resistive exercise and pamidronate: results from the LTBR study. Bone 2005; 36: 1019–1029.
33. Sipila S, Suominen H. Quantitative ultrasonography of muscle: detection of adaptations to training in elderly women. Arch Phys Med Rehabil 1996; 77: 1173–1178.
34. Williams PL, Berry MM, Collins P, Dyson M, Dussek JE, Ferguson MWJ, editors. Gray’s anatomy, the anatomical basis of medicine and surgery. 38th edn. New York: Churchill Livingstone; 1995.
35. Gibson JN, Smith K, Rennie MJ. Prevention of disuse muscle atrophy by means of electrical stimulation: maintenance of protein synthesis. Lancet 1988; 2: 767–770.