Heterotopic ossification is the formation of new bone in an abnormal location. It is usually seen following central nervous system disorders, including spinal cord injury, traumatic brain injury, encephalitis, and burn and trauma. Heterotopic ossification in post-stroke hemiplegia is rare; the reported incidence is 0.5–1.2%. It usually occurs on the paretic side of hemiplegic patients. We present here a case of post-stroke hemiplegia with heterotopic ossification in the non-paretic limb.
Key words: hemiplegia, stroke, heterotopic ossification.
J Rehabil Med 2007; 39: 500–502
Correspondence address: Zehra Kocaağa, Izmir Ataturk Eğitim ve Araştırma Hastanesi, 2. Fizik Tedavi ve Rehabilitasyon Kliniği, TR-35280 Yesilyurt, Izmir, Turkey.
E-mail: zehrakocaaga@hotmail.com
Submitted April 10, 2006; accepted March 5, 2007
INTRODUCTION
Heterotopic ossification (HO) is the formation of lamellar bone within the soft tissue surrounding a joint. It is usually seen after spinal cord injury (SCI), traumatic brain injury (TBI), burn, and direct trauma (1, 3). Although rare, HO can be seen in post-stroke hemiplegia. Varghese reported the incidence of HO after stroke as 0.5–1.2%.
The aetiopathogenesis of HO remains unclear. It has been hypothesized that the major aetiological factors are immobilization and forcible manipulation of joints to preserve range of motion (4, 5). Spasticity, fracture, infection, deep venous thrombosis and pressure ulcers are known to be contributing factors (6, 7).
HO is distinguished as traumatic and neurogenic. Traumatic HO may occur after direct muscle trauma or surgery (mainly following total joint arthroplasty). Neurogenic HO usually occurs following neurological lesions, such as brain injury or spinal cord injury (SCI) (8).
HO in post-stroke hemiplegia is rare; only a few cases have been reported in the literature. To our knowledge HO in the non-paretic limb has not been reported previously. We present here the case of a young woman who had right-sided post-stroke hemiplegia with HO in the non-paretic limb.
CASE REPORT
A 21-year-old hemiplegic woman was referred to our physical medicine and rehabilitation unit for rehabilitation.
Three months previously she had been admitted to a regional hospital with pre-eclampsia, whereafter a therapeutic Caesarean section was performed. At 7 days post-partum, right hemiplegia developed. Cranial computerized tomography (CT) was performed and a diffuse infarct was seen in the vascular supply to the middle cerebral artery. She was then referred to the neurology department of our hospital with a Glasgow Coma Scale (GCS) score of 8/15. A few days later the GCS had regressed to 3/15. She was immediately intubated and ventilated. CT scans of the brain were re-performed (10 days after the stroke) and revealed superior sagittal sinus venous thrombosis. After 2 weeks she was extubated and 2 months after the stroke, she was transferred to the neurology clinic from the intensive care unit with full co-operation and orientation. About 3 months later the patient was referred to our unit for rehabilitation, again with full co-operation and improved orientation.
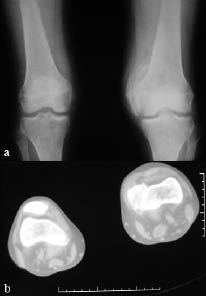
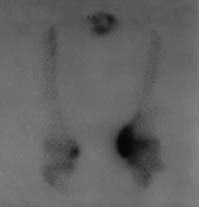
Neurological examination revealed findings of right hemiplegia. Motor recovery in the arm and leg was Brunnstrom stage II and III, respectively. According to the modified Ashworth scale (MAS), spasticity of the right wrist and finger flexors was grade 2 and spasticity of the gastrocnemius was grade 3. Active and passive range of motion of the non-paretic knee was restricted (range 40–90°). In addition, there was swelling and warmth on the left knee. Grade III pre-sacral pressure ulcer was present. Functional independence measure (FIMTM) was used for evaluation of the functional status of the patient; the FIMTM score was 54. Vital signs were within the normal ranges and no pathology was found in physical examinations of other systems. Her laboratory examination revealed a white blood cell count of 6000/mm3, erythrocyte sedimentation rate (ESR) of 97 mm/hour, serum alkaline phosphates (ALP) level 168 IU/l. Plain radiography and CT of the left knee showed calcification on the medial side of the femoral condyl, indicating HO (Fig. 1). Triple phase bone scan was performed and showed increased tracer uptake in both knees (Fig 2).
Fig. 1. (a) Plain radiography and (b) computed tomography (CT) of the left knee show calcification on the medial side of the femoral condyl, supporting a diagnosis of heterotopic ossification (HO).
Fig. 2. Triple phase bone scan of both knees. Tracer uptake is more pronounced in the non-paretic knee.
The hemiplegic rehabilitation of the patient mainly comprised exercise and transfer activities, balance and gait training for 2 months. Also, for treatment of the HO, cold and exercise therapy was performed for the left knee. Improvement of active and passive range of motion (ROM) of the left knee was achieved (range 0–110°). Motor recovery in the arm and leg was Brunnstrom stage V for both. Functional recovery was also achieved and the FIMTM score was increased to 121, which means independence in activities of daily living. Laboratory evaluations, including serum ALP level and ESR, were in the normal range at the last control visit. At the time of discharge, the patient ambulated independently without walking aids.
DISCUSSION
HO in post-stroke hemiplegia is very rare, only a few cases have been reported in the literature (9). The aetiopathogenesis of HO remains unclear. Some authors have proposed that neuronal control mechanisms are responsible for the aetiopathogenesis (10). Some authors have stated that a potential neurotransmitter may be responsible for this metabolic control. It is thought that HO, osteoporosis, Charcot joint, obesity- related increased bone density, etc. are conditions that are related to this control mechanism (11).
Some initiating or stimulating factors can be related to the neurogenic HO. These are: prolonged comatose stage, mechanical ventilation, increased muscle tone, and limited movement in the involved extremity (12, 13). The exact mechanisms of these factors relating to HO formation are not known. Our patient remained comatose for 2 months, and had mechanical ventilatory support for 2 weeks.
Besides these neurogenic stimulating factors, some authors have stated that immobilization, passive ROM exercises, transfer activities and repeated micro-traumas might be the chief contributors (4, 5, 6, 12, 14). Recent literature has stressed the hypothesis that HO might be traumatic in origin (15, 16). Michelsson et al. (4, 5) postulated that immobilization and forcible mobilization are the most important triggers of heterotopic bone formation. A histological study in rabbits has shown that HO occurs within 2–5 weeks following passive exercise of immobilized limbs. This model represents traumatic HO rather than modification of neurogenic HO pathways (4). Similarly, Ackerman (17). have revealed the same results in their experimental study. Some authors suggest that continued ROM exercises increase the inflammation and then increase the HO mass (18). In contrast, other authors have shown that ROM exercise maintains or increases joint mobility, but does not promote HO formation (19–21).
In our patient, during the comatose stage, passive ROM exercises were performed in both limbs equally, not only the paretic limb. However, this situation does not explain why HO occurred in the non-paretic limb. It is possible that if passive ROM exercises were performed less vigorously in the non-paretic limb this could have been a contributing factor for HO formation in that limb. As supported by the experimental studies, forced passive ROM exercises applied during long period of comatose stage in our patient may predispose to HO formation. During this comatose period, in addition to the passive ROM exercises, some transfer activities, poor positioning or hygienic care might have caused micro-trauma or inflammation that resulted in HO. Also, in our opinion, long periods of coma and mechanical ventilation might be contributing factors for developing HO. Therefore, in our case, both neurogenic and traumatic factors together might have had a role in HO formation in the non-paretic and the paretic limb.
The presence of HO may increase disability, which is also present because of the original disease. It is therefore important to prevent HO formation, and, if it has occurred, it must be recognized early and treated appropriately. Although there are controversial studies regarding the use of ROM exercises in the treatment protocol (4, 17, 19–21), gentler exercising within the pain-free range can be more useful for these patients. In addition, further human studies related to these treatment modalities are required.
REFERENCES
1. Lal S, Hamilton BB, Heinemann A, Betts HB. Risk factors for heterotopic ossification in spinal cord injury. Arch Phys Med Rehabil 1989; 70: 387–390.
2. Varghese G. Heterotopic ossification. Phys Med Rehabil Clin North Am 1992; 3: 407–415.
3. Warren SB. Heterotopic ossification after total hip replacement Orthop Rev 1990; 19: 603–611.
4. Michelsson JE, Rauschning W. Pathogenesis of experimental heterotopic bone formation following temporary forcible exercising of immobilized limbs. Clin Ortop 1983; 176: 265–272.
5. Michelsson JE, Ganroth G, Andersson LC. Myositis ossificans following forcible manipulation of the leg. A rabbit model for the study of heterotopic bone formation. J Bone Joint Surg Am 1980; 62: 811–815.
6. Garland DE. A clinical perspective on common forms of acquired heterotopic ossifications. Clin Ortho Rel Res 1991; 263: 13–29.
7. Stover SL. Heterotopic ossification after spinal cord injury In: Bloch RF, Bascaum M, editors. Management of spinal cord injuries. Baltimore: William & Wilkins; 1986, p. 284–301.
8. Johns JS, Cifu DX, Keyser-Marcus L, Jolles PR, Fratkin MJ. Impact of clinically significant heterotopic ossification on functional outcome after traumatic brain injury. J Head Trauma Rehabil. 1999; 14: 269–276.
9. Chua KSG, Kong KH. Acquired heterotopic ossification in the settings of cerebral anoxia and alternative therapy: two cases. Brain Inj. 2003; 17: 535–544.
10. Garland DE. Heterotopic ossification. In: Nickell VL, Botte MJ, editors. Orthopedic rehabilitation. New York: Livingstone; 1992, p. 453– 469.
11. Jones KB, Mollano AV, Morcuende JA, Cooper RR, Saltzman CL. Bone and brain: a review of neural, hormonal, and musculoskeletal connections. Iowa Orthop J 2004; 24: 123–132.
12. Whyte J, Hart T, Laborde A, Rosenthal M. Rehabilitation issues in traumatic brain injury. In: DeLisa JA, editor. Physical medicine and rehabilitation: principles and practice. Vol II Philedelphia: LWW; 2005, p. 1677–1715.
13. Zeilig G, Weingarden HP, Levy R, Peer I, Ohry A, Blumen N. Heterotopic ossification in Guillain-Barre syndrome: incidence and effects on functional outcome with long-term follow-up. Arch Phys Med Rehabil 2006; 87: 92–95.
14. Bosse A. Clinical aspects, differential diagnosis and histogenesis of heterotopic ossifications. Veroff Pathol 1997; 146: 1–168.
15. Lotta S, Scelsi L, Scelsi R. Microvascular changes in the lower extremities of paraplegics with heterotopic ossification. Spinal Cord 2001; 39: 595–598.
16. Snoecx M, De Muynck M, Van Laaere M. Association between muscle trauma and heterotopic ossification in spinal cord injured patients: reflections on their causal relationship and the diagnostic value of ultrasonography. Paraplegia 1995; 33: 464–468.
17. Ackerman LV. Extraosseous localized non-neoplastic bone and cartilage formation (so-called myositis ossificans): clinical and pathological confusion with malignant neoplasms. J Bone Joint Surg Am 1958; 40A: 279–298.
18. Furman R, Nicholas JJ, Jivoff L. Elevation of serum alkaline phosphatase coincident with ectopic bone formation in paraplegic patients. J Bone Joint Surg Am 1970; 52: 1131–1137.
19. Garland DE, Shimoyama ST, Lugo C, Barras D, Gilgoff I. Spinal cord insults and heterotopic ossification in the pediatric population. Clin Orthop 1989; 245: 303–310.
20. Stover SL, Hataway CJ, Zeiger HE. Heterotopic ossification in spinal cord injured patients. Arch Phys Med Rehabil 1975; 56: 199–204.
21. Wharton GW, Morgan TH. Ankylosis in the paralyzed patients. J Bone Joint Surg Am 1970; 52: 105–112.