OBJECTIVE: To study the feasibility and 1-year effects on subjective health and symptoms of a network-based geriatric rehabilitation intervention for frail elderly people.
DESIGN: A randomized, controlled trial.
SUBJECTS: A total of 741 frail elderly people who lived at home, aged > 65 years, without severe cognitive impairment (Mini Mental State Examination < 18), and eligible to receive Pensioners’ Care Allowance (a benefit that is independent of personal income or insurance). The setting included 41 municipalities and 7 rehabilitation centres in Finland.
METHODS: Over a period of 8 months the intervention group received network-based rehabilitation for 3 in-patient periods (totalling 21 days) at rehabilitation centres and a home visit by a professional. Both groups received standard social and health services locally. Functional Independence Measure, subjective health, common symptoms and pain were assessed at baseline and 1-year follow-up.
RESULTS: After baseline measurements, 33 of those allocated to rehabilitation withdrew from the study. Of the 343 intervention subjects, 276 attended all 3 in-patient periods. At one year, there were no differences in symptoms between the groups. Subjective health was improved in the intervention group and impaired in the control group (p < 0.01).
CONCLUSION: The network-based geriatric rehabilitation programme was feasible for use among the frail elderly people and improved their subjective health.
Key words: frail elderly people, randomized controlled trial, rehabilitation, feasibility study, health status, rehabilitation centre.
J Rehabil Med 2007; 39: 473–478
Correspondence address: Katariina Hinkka, Social Insurance Institution, Research Department, Peltolantie 3, FI-20720 Turku, Finland. E-mail: katariina.hinkka@kela.fi
Submitted March 31, 2006; accepted February 14, 2007
INTRODUCTION
The most prominent global population trend at present is the rapid increase in the older age groups, especially the oldest old (1). In terms of both health and economy, frail elderly people pose a great challenge to all developed societies, independent of national differences in service systems. Autonomous living at home for as long as possible has become a public health priority, and effective measures to prevent or delay disability and institutionalization are urgently called for (2, 3).
Frail elderly people are a high-risk group for adverse health events, including disability, dependency, falls, need for long-term care, and mortality (4). Among community-living elderly people, frailty often progresses insidiously (5, 6). The great majority of geriatric rehabilitation measures have focused on specific geriatric conditions resulting from acute medical events, such as stroke or hip fracture. Non-specific frailty has rarely been addressed in geriatric rehabilitation. Recently, a need for more preventive and rehabilitative approaches has been expressed (7, 8).
In non-specific progressive frailty, there is no consensus on what types of interventions are feasible and whom they benefit most, or whether such interventions can delay institutionalization (9–12). A rehabilitation programme targeted at frail elderly people and designed to support independent living has been in place in Finland since the year 2000. Financed by the Social Insurance Institution of Finland (SII), the programme is based on the networking of institutional rehabilitation providers and local social and health services. It aims to increase elderly people’s functional capacity and functional ability so as to enable them to live in the community as independently as possible.
The “AGE” study is a national randomized long-term multi-centre project aimed at evaluating this new network-based geriatric rehabilitation programme in comparison with the use of standard health and social services (13). The research project includes several sub-studies to ensure a multifaceted approach with quantitative and qualitative research methods. The main hypothesis is that frail elderly people participating in the rehabilitation intervention will be able to live at home longer than the control group. This paper describes the subjects’ attendance and reasons for dropping out of the intervention, and compares the subjects participating in the multidisciplinary, active rehabilitation programme with those in usual care, for the following perceptive measures: health, common symptoms, pain and exhaustion.
METHODS
Inclusion and exclusion criteria
The eligibility criteria were selected to allow the identification of frail elderly people who are at high risk of institutionalization, but who have the cognitive and physical potential necessary for participating in the intervention.
To be eligible, the people were required to be over 65 years of age, to have progressively decreasing functional capacity, and to be living at home, but having their coping at home threatened and a projected risk of institutionalization within 2 years. As an objective indicator of frailty, they had to meet the criteria of entitlement for Pensioners’ Care Allowance. Pensioners’ Care Allowance is a benefit granted by the SII to compensate for the costs for the person’s care at home. It is granted to people with a medical disability verified by a physician, and a need of assistance (14). The allowance is intended to cover the costs of assistance, and it is independent of the pensioner’s income or insurance.
Exclusion criteria included acute or aggressively proceeding diseases that would prevent participation in the rehabilitation, severe cognitive impairment (Mini Mental State Examination (MMSE) < 18 points), or participation in an in-patient rehabilitation during the preceding 5 years.
Selection process
Subjects were enrolled during the year 2002 in a 2-phase selection process. Initially, potential participants were recruited in 42 municipalities by local social and health care officials, who had received specific instructions accompanied by written guidelines. In the second phase, the representatives of the municipality, the rehabilitation centre concerned and the local SII office jointly assessed the selected people’s eligibility and suitability for rehabilitation. The aim was to find 18 people from each municipality to be randomized into intervention subjects (n = 8), controls (n = 8) and substitutes (n = 2).
Randomization and formation of study groups
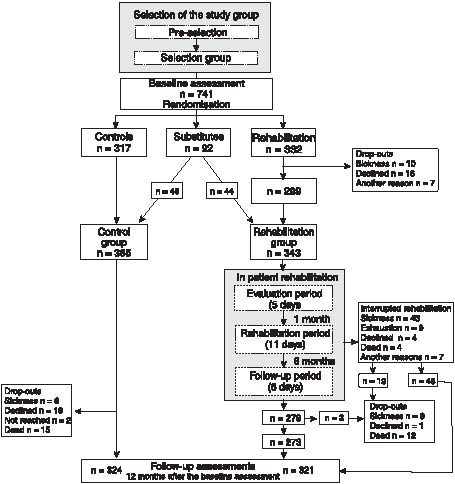
A total of 741 elderly people were recruited, i.e. 13–18 people per municipality. Following baseline measurements, they were allocated randomly to the intervention (n = 332), control (n = 317) and substitute (n = 92) groups using numbered and sealed envelopes stratified by gender. Each municipality had its own envelopes containing, in random order, an allocation to one of the 3 groups. Subjects in the intervention group were referred to geriatric rehabilitation at a rehabilitation centre in groups of 8 people, while the control group received standard social and health care services. If any person in the intervention group declined to participate in the rehabilitation, they were replaced (Fig. 1).
Fig. 1. Study participants.
Of the subjects in the intervention group, 33 dropped out and were replaced. An additional 11 substitutes completed the rehabilitation groups, while the rest of the substitutes were integrated into the control group. Thus, the final study group consisted of 343 people in the intervention group (IG) and 365 in the control group (CG) (Fig. 1).
The ethics committees of the SII and Turku University Hospital approved the study protocol. All of the study participants provided written informed consent.
Intervention
Subjects in the intervention group participated in a multidisciplinary, active geriatric rehabilitation programme. The controls relied on standard, locally provided social and health care services. The rehabilitation programme was based on networking between the various actors: the rehabilitation institutes, the local social and health service providers, the SII and various non-governmental organizations (e.g. patient organizations, the Church). It aimed at increasing the elderly people’s ability to live independently in the community. The multidisciplinary model was based on current knowledge of geriatric rehabilitation (1, 9, 15–17) and was developed on the basis of the resources and geriatric expertise accumulated over the years at rehabilitation centres that provide extensive rehabilitation for World War II veterans (18).
Briefly, the rehabilitation programme was based on a group rehabilitation approach and managed by a multidisciplinary geriatric rehabilitation team. Individual and group-based activities were implemented at the rehabilitation centres. The rehabilitation programme consisted of 3 in-patient periods (5, 11 and 5 days) within a period of 8 months.
The first period and the follow-up period were individually oriented. During the first period, a comprehensive geriatric assessment took place and the participants were introduced to the aims and contents of the rehabilitation programme. The key members of the rehabilitation team (e.g. physician, physiotherapist, social worker, occupational therapist) met personally with each participant. In addition, they had group activities, which in most cases involved physical activity.
A home visit by an occupational or physical therapist from the rehabilitation centre, together with a representative of the municipality, took place prior to the second period about one month later. The second period was a multidisciplinary group-based intervention that included physical, psychological and social activation and counselling, motivating the participants to adopt an active lifestyle, classes on disease management and coping strategies, and recreational activities. The main part of group activities focused on physical activation. The participants were encouraged to invite their personal caregiver (family member or friend) to accompany them for 3 days at the end of this period. An individualized plan for future rehabilitation activities and care was devised for each participant by the rehabilitation team, in co-operation with the patient and a municipal officer.
The follow-up period took place 6 months after the first rehabilitation period. The objective of this period was to refresh the instructions given during the earlier periods and to adjust the home-training regimen, if necessary.
Measurements
All measurements were carried out at the healthcare centres of the participants’ home municipalities by 3 examiners, who were qualified physical therapists, extensively trained for the assessments and without any role in the intervention.
Questionnaires were sent in advance to each participant who brought them along to the health centre. Questionnaires were used to obtain data concerning the participants, their current health status, functional capacity, living conditions, use of walking aids and other assistive devices, and care providers. The questionnaires were checked by the examiner on arrival at the health centre and any incomplete sections were completed by interviewing the participants.
Perceived health at present was measured on a 5-point Likert scale (ranging from “very good” to “very poor”). Present pain was assessed on the Visual Analogue Scale (VAS; 19), ranging from “no pain” (0) to “maximal pain” (100). Scores were measured to the nearest millimetre with a ruler. A change was defined as 5 mm or more.
A 5-point Likert scale was used for assessment of common symptoms. Symptoms included 4 questions about pain concerning headache, chest pain, abdominal pain or nausea, and constant pain or aching. Difficulty breathing and feelings of powerlessness or vertigo were recorded. An index (Symptom Index) was formed of the said 6 symptoms by adding up the points (0 = no symptoms, 1 = little, 2 = some, 3 =much or very much). The total score thus varied from 0 to 18.
To assess the participants’ cognitive status the MMSE; 20 was used with a scale from 1 to 30; the lower scores indicate reduced cognitive capacity.
Data about the participants’ functional ability (Functional Independence Measure (FIM™ 5.0; 21) and mood (Geriatric Depression Scale (GDS-15); 22) were collected and mobility (the 20-feet (6.1 m) walking speed test; 23, chair rise test; 24), handgrip strength (Jamar dynamometer, Sammons™ Preston, Canada), and respiratory function (Peak Expiratory Flow, PEF) were measured by the examiners.
At 12 months, the subjects in the IG were asked to assess their satisfaction with the rehabilitation on a 5-point Likert scale (range “very satisfied” to “very unsatisfied”).
Statistical methods
The data were analysed using SAS version 8.02 software (Statistical Analysis System 1999–2001). Differences between the IG and CG groups, as well as between the attendants and drop-outs were tested using Pearson’s χ2 test or Fisher’s exact test. Student’s t-test was used to compare numeric variables. Differences in the changes between the groups were tested with an analysis of variance for repeated measures or comparing marginal distributions of categorical variables using the SAS CATMOD procedure. p-values below 0.05 were taken as evidence of statistical significance.
RESULTS
Baseline characteristics of the study groups
At baseline, the mean age of the subjects was 78.4 years (range 65–96 years). A majority of subjects were female (86%), widowed or single, living alone in an urban area, and perceiving health deterioration (66%) during the preceding year. The baseline characteristics of the study groups are shown in Table I. The differences between the IG and the CG were insignificant at baseline.
| Table I. Baseline characteristics of the intervention group (IG) and the control group (CG). |
| Variables | IG (n = 343) | CG (n = 365) | p-value* |
| Categorical variables (%) |
| Living arrangements | | | |
| Household members | | | |
| Living alone | 75.0 | 70.1 | 0.15 |
| Living with spouse | 16.0 | 19.5 | 0.23 |
| Living with children | 9.0 | 9.9 | 0.70 |
| Form of dwelling | | | |
| Living independently at home | 41.4 | 42.7 | |
| Living at home assisted by home care | 42.6 | 40.8 | 0.89 |
| Living in a service accommodation | 16.0 | 16.5 | |
| Source of assistance† | | | |
| Social services | 81.4 | 81.4 | 0.99 |
| Informal help | | | |
| Family members | 81.7 | 79.5 | 0.45 |
| Friends and neighbours | 28.2 | 26.9 | 0.69 |
| Use of walking aids | 73.5 | 73.7 | 0.95 |
| Numeric variables, mean (SD) | | | |
| Functional capacity (FIM) | | | |
| Self care (range 8–56) | 50.5 (4.9) | 50.3 (5.4) | 0.68 |
| Mobility (range 5–35) | 31.3 (2.8) | 31.0 (3.2) | 0.13 |
| Cognition (range 5–35) | 34.1 (1.7) | 34.1 (1.9) | 0.91 |
| Physical capacity | | | |
| Fast walking (20-feet) | | | |
| Speed (m/sec) | 1.55 (0.90) | 1.55 (0.92) | 0.98 |
| Number of steps (n) | 14.2 (4.9) | 14.3 (5.0) | 0.93 |
| 5 repetitive chair stand-ups (sec)‡ | 25.1 (11.0) | 23.4 (8.9) | 0.041 |
| Grip strength (kg) | | | |
| Right hand | 21.8 (7.8) | 21.9 (8.2) | 0.96 |
| Left hand | 20.5 (7.9) | 20.3 (8.0) | 0.64 |
| Peak expiratory flow (l/min) | 301.5 (92.2) | 301.8 (90.5) | 0.963 |
| Cognition | | | |
| Cognitive function (MMSE) | 25.3 (2.9) | 25.1 (2.9) | 0.36 |
| Depressive mood (GDS) | 4.1 (2.5) | 4.2 (2.5) | 0.63 |
| *Significance between the groups. †Assistance may be received from multiple sources. ‡IG (n = 278), CG (n = 276). FIM: Functional Independence Measure; MMSE: Mini Mental State Examination; GDS: Geriatric Depression Scale. |
The main diagnostic categories for the SII Pensioners’ Care Allowance were cardiovascular diseases in 32% of subjects, musculoskeletal diseases in 29% and mental and nervous diseases in 15%, with no difference observed in the prevalence between the groups. Depressive mood (GDS = 7–13 points) was found in 17% of subjects and declined cognitive capacity (MMSE < 24 points) in 28%.
Participation and dropping out
Refusal to participate. A drop-out analysis was conducted, covering the people who refused to participate after being randomized to the intervention. Age, marital status, geographical location, residential environment, type of dwelling, subjective health, cognitive status (MMSE), depressive symptoms (GDS) or the FIM™ subscales did not differentiate the drop-outs (n = 33) from the intervention group (n = 343).
Interruption of the intervention. Of the 343 IG subjects who started the rehabilitation intervention, 67 discontinued before the end of the final in-patient period (Fig. 1). Thus, the final participation rate was 81%. Reasons for interruption were sickness, including 2 accidents (64%), exhaustion and general functional decline (13%), refusal (6%) and death (6%). Dissatisfaction with rehabilitation was the reason for the withdrawal in 3 cases, and the husband’s opposition in 2 cases. One person’s programme was interrupted by the staff because of her inability to cope cognitively in the rehabilitation centre setting. For one person, no reason was available.
Subjects were recorded as having interrupted the programme even if they later returned to it. For example, 7 subjects interrupted the programme during the first in-patient period, but 2 of them continued to participate in the second period. Of these, one dropped out for good during the second period, while the other was able to complete the programme.
Most of the interruptions (n = 42; 63%) occurred between the second and third inpatient periods (Fig. 1). The drop-outs perceived their health as worse (p < 0.0001), had a lower lung function (p = 0.005), were older (p = 0.008) and more depressed (p = 0.043) at baseline than the rest of the people in the IG, who completed all the 3 inpatient rehabilitation periods (Table II). However, the participation rate was high in the oldest age quartile (78%), in people with the lowest quartile of PEF (74%), in people with the highest quartile of GDS score (77%) and in people with poor subjective health (65%).
| Table II. Non-completers (drop-outs; n = 67) vs the rest of the intervention group who completed all 3 in-patient periods (n = 276). Values are expressed as means (standard deviation) unless otherwise indicated. |
| Variable | Drop-outs (n = 67) | Full attendance (n = 276) | Group comparisons p |
| Gender (female %) | 88 | 84 | 0.38 |
| Age (years) | 80.0 (5.7) | 77.6 (6.68) | 0.008 |
| FIM™ self care (score) | 50.3 (4.2) | 50.5 (5.0) | 0.76 |
| FIM™ mobility (score) | 31.1 (2.9) | 31.4 (2.8) | 0.60 |
| FIM™ cognition (score) | 33.9 (2.0) | 34.1 (1.7) | 0.49 |
| Walking, speed (m/sec) | 1.62 (0.99) | 1.53 (0.98) | 0.47 |
| Walking, steps (n) | 15.0 (5.7) | 14.1 (4.6) | 0.18 |
| 5 rep stand-ups (sec) | 25.3 (10.45) | 25.09 (11.11) | 0.92 |
| Hand grip, right (kg) | 21.2 (7.0) | 22.0 (8.0) | 0.42 |
| Hand grip, left (kg) | 19.7 (6.9) | 20.8 (8.1) | 0.33 |
| Peak expiratory flow (l/min) | 273.5 (84.0) | 308.9 (93.8) | 0.005 |
| Walking aids (%) | 80.6 | 71.7 | 0.14 |
| MMSE (points) | 25.0 (3.2) | 25.4 (2.8) | 0.32 |
| Living alone (%) | 70.2 | 76.1 | 0.31 |
| Living with spouse (%) | 20.9 | 15.2 | 0.26 |
| Living with children (%) | 9.0 | 8.7 | 0.95 |
| Form of dwelling (%)1 | 43/45/12 | 41/42/17 | 0.59 |
| Social services (%) | 82.1 | 80.8 | 0.81 |
| Assistance (family, %) | 91.0 | 79.4 | 0.027 |
| Assistance (friends, %) | 26.9 | 28.6 | 0.77 |
| Private home-care (%) | 23.9 | 18.8 | 0.35 |
| Subjective health (%)2 | 3/45/52 | 4/72/24 | < 0.0001 |
| Pain VAS (mm) | 41 (29) | 42 (27) | 0.94 |
| Symptom Index (score) | 8.81 (3.60) | 8.04 (3.71) | 0.13 |
| GDS (points) | 4.66 (2.53) | 3.97 (2.45) | 0.043 |
| 1Forms of dwelling: living independently at home / living at home assisted by home care / living in a service accommodation. 2Measured on a 5-point Likert scale, alternatives “very good” or “good” / “average” / “poor” or “very poor”. FIM™: Functional Independence Measure; MMSE: Mini Mental Scale Examination; VAS: Visual Analogue Scale; GDS: Geriatric Depression Scale. |
Adverse events. Adverse events during the inpatient periods resulting in an interruption of the period included 2 accidents, one of which was a shoulder injury during muscle strength training and the other was a fall at the entrance of the institution. Additionally, one fall occurred between the inpatient periods (Fig. 1). Some occasional days off from the rehabilitation programme took place because of diverse ailments.
Participation in follow-up measurements. A total of 645 (91%) people attended the 1-year follow-up measurements (95% of the people alive at that time). The participation rate was 94% in the IG and 89% in the CG (97% and 92.5% of the people alive, respectively). Of those participants in the IG who interrupted the intervention, 48 (72%) attended the follow-up measurements and thus data concerning them were available for the analyses (Fig. 1).
Mortality, institutionalization and functional independence
By the 1-year follow-up, 27 people (4%) had died (12 in the IG and 15 in the CG). Four percent of subjects in both groups had been institutionalized. The total FIM™ score had decreased in both groups, –0.32 (SD 0.49) in the IG and –0.38 (SD 0.63) in the CG (p = 0.0579 between the groups). Concerning the FIM™ subscales, differences favoured the IG, but were not statistically significant. FIM™ mobility decreased from 6.29 (SD 0.54) to 5.96 (SD 0.65) points in the IG and from 6.23 (SD 0.61) to 5.85 (SD 0.81) points in the CG. FIM™ self-care decreased from 6.35 (SD 0.59) to 6.15 (SD 0.75) points and from 6.30 (SD 0.67) to 6.09 (SD 0.84) points, and FIM™ cognition from 6.84 (SD 0.30) to 6.74 (SD 0.37) points in the IG and from 6.82 (SD 0.33) to 6.69 (SD 0.51) points in the CG, respectively.
Perceptions 3 months after rehabilitation
By the 1-year follow-up, 3 months had passed since the end of the final rehabilitation period. At this point, 93% of participants in the IG reported being very satisfied or satisfied with the rehabilitation, 4% were unsatisfied, and 3% had no opinion.
In the IG, subjective health improved (p = 0.0337) while in the CG it decreased (p = 0.0193). The change from baseline to follow-up was significantly different between the groups for subjective health (p = 0.0255) (Table III).
For pain, no significant differences between the groups were observed. By the 1-year follow-up, pain decreased in both groups but only in the IG was the decrease significant (p = 0.0456) (Table III).
| Table III. Subjective health and pain in the intervention group (IG) and control group (CG). Given as percentages for the scores of subjective health, and means and standard deviations (SD) for pain, measured by VAS (mm). |
| | Baseline | Follow-up | Change pa | Group by time interaction pb |
| Subjective health (%) |
| IG (n = 321) Very good or good Average Poor or very poor | 3.7 68.2 28.0 | 9.0 60.1 30.9 | 0.0017 | 0.026 |
| CG (n = 322) Very good or good Average Poor or very poor | 4.7 66.1 29.1 | 4.7 57.8 37.6 | 0.019 |
| p between groupsc | 0.76 | 0.034 | | |
| Pain (mean (SD)) |
| IG (n = 314) | 42 (27) | 38 (29) | 0.046 | 0.64 |
| CG (n = 313) | 40 (31) | 38 (30) | 0.21 |
| p between groupsc | 0.46 | 0.81 | | |
| aDifference between baseline and follow-up. bDifference between the changes in IG and CG. cDifference between IG and CG. VAS: Visual Analogue Scale. |
In the Symptom Index, no significant between-group or within-group effects at the 1-year follow-up were observed. Individual questions concerning pain, breathing and exhaustion did not reveal any differences between the groups at one year.
The mean MMSE decreased by 0.4 (SD 3.0) points in the IG and 0.9 (SD 3.4) points in the CG (p = 0.054). At the follow-up, people with a baseline MMSE score < 24 points did not differ from those with a normal MMSE score for the measures of pain, symptoms or subjective health.
DISCUSSION
This study showed that it is feasible to design and carry out a large-scale multifaceted network-based rehabilitation intervention for frail elderly people. Despite the fact that the participants were frail and had a high risk of institutionalization, most of them were able to carry out all 3 in-patient periods of the rehabilitation programme. The programme prevented deterioration of subjective health, a global measure of disease burden and well-being. However, due to small cell sizes of different complaints in the context of frailty, it was difficult to show improvement in any single symptom, such as pain.
The target group of the present study consisted of the frailest elderly people living in the community, who were selected on the basis of their increased risk of institutionalization. Consequently, specific diagnoses were not used as inclusion criteria. The aim was to slow down the gradual progression of disability and thus to delay institutionalization (25). The study was a randomized “geriatric evaluation and management” trial with a multidisciplinary approach to frailty. As far as we know, there are no earlier research reports concerning rehabilitation targeted to frail elderly people with no specific diagnoses.
Among frail elderly people, adherence may be a major issue in interventions of this kind, and consequently, an annual drop-out rate of up to 20% is considered acceptable (3, 26). Given the high age and frailty of our participants, the attrition rate was low in our study. Approximately 90% of those allocated to rehabilitation participated in the first in-patient period and 81% were able to complete all 3 in-patient periods. Prior to the first period, most of the withdrawals were due to refusals. During the course of the programme, acute illness was the main cause for interrupting the programme. The drop-outs were older, had poorer subjective health and more depressive symptoms than those who continued in the programme. Impaired cognitive capacity did not predict lower attendance, adverse events or increased symptoms. It has also been observed previously that cognitively impaired patients respond to geriatric rehabilitation in the same way as cognitively intact patients (27–29). The contents of our intervention were in line with preventive approaches introduced in rehabilitation and geriatric care of frail elderly people (7, 30). Overall, the majority of participants in the current study were able to cope successfully with an active rehabilitation intervention in a rehabilitation centre setting, away from home. No increases in symptoms, pain or exhaustion could be attributed to the intervention. Adverse events during the rehabilitation periods were scarce. In summary, it is our experience that in-patient intervention among frail elderly people is feasible.
The intervention increased the proportion of those reporting good self-rated health and, at the same time, prevented the increase in the proportion of those with poor self-rated health. Subjective or self-rated health is a global measure of health. An individual evaluates health-related aspects in relation to different contextual frames, such as one’s age and what is considered normal at that age (31). Poor and fair self-rated health reflect burden of illness, while the higher levels of self-rated health reflect well-being and functional capacity (32). Unfortunately, we were unable to analyse the mechanisms underlying the changes in self-rated health. It is possible that self-rated health improved as a consequence of improved subjective well-being. However, in the IG group some individuals may have compared themselves with other participants in their group who may have been younger but equally ill. A change in one’s reference in terms of what is typical for people of matching age may also improve one’s view of one’s own health. Nevertheless, self-rated health is considered a valid predictor of future changes in health (33), and therefore may be interpreted to indicate improvement in the IG group.
The level of satisfaction with the rehabilitation was very high; 93% of the participants reported being satisfied or very satisfied. Elderly respondents, in particular, tend to report high levels of satisfaction in questionnaires. Processes of transformation from negative perceptions to positive summary assessments have been identified (34). Thus, certain scepticism may be justified concerning the satisfaction results. In addition, the functional outcome, i.e. the FIM™ score, decreased in both groups. However, certain limitations in the applicability of the FIM™ in settings other than acute care have been reported previously (35).
The design of the present study fulfilled the consensus report criteria published by Ferrucci et al. (3) on designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail elderly people. A weakness of the study, however, is the fact that the intervention was implemented at 7 different rehabilitation centres. Each centre was guided by common standards and guidelines, but they implemented rehabilitation on the basis of their own resources and long-term expertise in geriatric rehabilitation.
At this phase of the study it can be concluded that, in terms of attendance and adherence to the intervention, the feasibility of the programme was good. The symptoms and level of exhaustion of the intervention participants did not increase and their subjective health improved in comparison with the controls. Future follow-up studies will show whether and to what extent and at what cost the programme decelerates functional decline and delays institutionalization of frail elderly people.
REFERENCES
1. World population prospects: the 2004 revision. New York: Department of Economic and Social Affairs. Population Division, United Nations; 2005.
2. Heikkinen E. What are the main risk factors for disability in old age and how can disability be prevented. Health Evidence Network (HEN) synthesis report on main risk factors for disability in old age and how can disability be prevented. Copenhagen: WHO; 2003.
3. Ferrucci L, Guralnik JM, Studenski S, Fried LP, Cutler GB, Walston JD. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriatr Soc 2004; 52: 625–634.
4. Hogan D, MacKnight C, Bergman H. Models, definitions, and criteria of frailty. Aging Clin Experim Res 2003; 15 Suppl 3: 3–29.
5. Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol Med Sci 2004; 59: M255–M263.
6. Gill TM, Allore H, Holford TR, Guo Z. The development of insidious disability in activities of daily living among community-living older persons. Am J Med 2004; 117: 484–491.
7. Hallberg IR, Kristensson J. Preventive home care of frail older people: a review of recent case management studies. J Clin Nurs 2004; 13: 112–120.
8. Gill T, Baker D, Gottschalk M, Peduzzi P, Allore H, Byers A. A program to prevent functional decline in physically frail, elderly persons who live at home. N Engl J Med 2002; 14: 1068–1074.
9. Jonsson A, Gustafson Y, Schroll M, Hansen F, Saarela M, Nygaard H, et al. Geriatric rehabilitation as an integral part of geriatric medicine in the Nordic countries. Dan Med Bull 2003; 50: 439–445.
10. Wieland D. The effectiveness and costs of comprehensive geriatric evaluation and management. Crit Rev Oncol Hematol 2003; 48: 227–237.
11. Rockwood K, Stadnyk K, Carver D, MacPherson KM, Beanlands HE, Powell C, et al. A clinimetric evaluation of specialized geriatric care for rural dwelling, frail older people. J Am Geriatr Soc 2000; 48: 1080–1085.
12. Kuo H-K, Scandrett KG, Dave J, Mitchell SL. The influence of outpatient comprehensive geriatric assessment on survival: a meta-analysis. Arch Gerontol Geriatr 2004; 39: 245–254.
13. Hinkka K, Karppi S-L, Aaltonen T, Ollonqvist K, Grönlund R, Salmelainen U, et al. A network-based geriatric rehabilitation programme: study design and baseline characteristics of the patients. Int J Rehab Med 2006; 29: 97–103.
14. Kela 2004. Pensioners’ care allowance [on-line]. Available from: http://www.kela.fi/in/internet/english.nsf/NET/110806122436AK? openDocument. [accessed October 25, 2006].
15. Wells JL, Seabrook JA, Stolee P, Borrie MJ, Knoefel F. State of art in geriatric rehabilitation. Part I: review of frailty and comprehensive geriatric assessment. Arch Phys Med Rehabil 2003; 84: 890–897.
16. Helander E, editor. Prejudice and dignity. An introduction to community-based rehabilitation. United Nations Development Programme. Second edn. New York: United Nations; 1999.
17. WHO. Active ageing. Second United Nations World Assembly on Ageing. Madrid: WHO; 2002.
18. Ollonqvist K, Grönlund R, Karppi S-L, Salmelainen U, Poikkeus L, Hinkka K. A network-based rehabilitation model for frail elderly people – development and assessment of a new model. Scand J Caring Sci 2006 (in press).
19. Huskinsson EC. Measurement of pain. Lancet 1974; II: 1127.
20. Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the state of patients for the clinician. J Psychiatric Res 1975; 12: 189–198.
21. Granger CV, Hamilton BB, Keith RA, Zielezny M, Sherwin FS. Advances in functional assessment for medical rehabilitation. Top Geriatr Rehabil 1986; 1: 59–74.
22. Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey MB, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatri Res 1983; 17: 37–49.
23. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol Med Sci 1994; 49: M85–M94.
24. Guralnik JM, Seeman TE, Tinetti ME, Newitt MC, Berkman LF. Validation and use of performance measures of functioning in a non-disabled older population: MacArthur studies of successful ageing. Aging Clin Exp Res 1994; 6: 410–419.
25. Ferrucci L, Guralnik JM, Simonsick E, Salive ME, Corti C, Langlois J. Progressive versus catastrophic disability: a longitudinal view of the disablement process. J Gerontol Med Sci 1996; 51A: M123–M130.
26. Aminzadeh F. Adherence to recommendations of community-based comprehensive geriatric assessment programmes. Age Ageing 2000; 29: 401–407.
27. Diamond PT, Felsenthal G, Macciocchi SN, Butler DH, Lally- Cassady D. Effect of cognitive impairment on rehabilitation outcome. Am J Phys Med Rehabil 1996; 75: 40–43.
28. Teri L, Gibbons LE, McCurry SM, Logsdon RG, Buchner DM, Barlow WE, et al. Exercise plus behavioural management in patients with Altzheimer disease: a randomized controlled trial. JAMA 2003; 290: 2015–2022.
29. Huusko T, Karppi P, Avikainen V, Kautiainen H, Sulkava R. Randomized clinically controlled trial of intensive geriatric rehabilitation in patients with hip fracture: subgroup analysis of patients with dementia. BMJ 2000; 321: 1107–1111.
30. Gill TM, Baker DI, Gottschalk M, Peduzzi PN, Allore H, Van Ness PH. A prehabilitation program for the prevention of functional decline: effect on higher-level physical function. Arch Phys Med Rehabil 2004; 85: 1043–1049.
31. Jylhä M, Guralnik J, Balfour J, Fried L. Walking difficulty, walking speed, and age as predictors of self-rated health: the Women’s Health and Ageing Study. J Gerontol Med Sci 2001; 10: M609–M617.
32. Benyamini Y, Leventhal EA, Leventhal H. Elderly people’s ratings of the importance of health-related factors to their self-assessments of health. Soc Sci Med 2003; 56: 1661–1667.
33. Pinquart M. Correlates of subjective health in older adults: a meta-analysis. Psychol Aging 2001; 16: 14–26.
34. Edwards C, Staniszweska S, Crichton N. Investigation of the ways in which patients’ reports of their satisfaction with healthcare are constructed. Sociol Health Illn 2004; 26: 159–183.
35. Jette A, Haley S. Contemporary measurement techniques for rehabilitation outcomes assessment. J Rehabil Med 2005; 37: 339–345.