EVALUATION OF BOTULINUM TOXIN THERAPY OF SPASTIC EQUINUS IN PAEDIATRIC PATIENTS WITH CEREBRAL PALSY
EVALUATION OF BOTULINUM TOXIN THERAPY OF SPASTIC EQUINUS IN PAEDIATRIC PATIENTS WITH CEREBRAL PALSY
Paolo Manganotti, MD, PhD, Fabio Zaina, MD, Maurizio Falso, MD, Franco Milanese, MD and Antonio Fiaschi, MD
From the Section of Neurological Rehabilitation, Department of Neurological Sciences and Vision, University of Verona, Verona, Italy
OBJECTIVE: To develop a clinical and instrumental protocol to assess the postural and dynamic effects following treatment with botulinum neurotoxin A in children with cerebral palsy affected by spastic equinus.
DESIGN: Open study, in which every patient served as his or her own control.
Patients: Ten sequential children with cerebral palsy and spastic dynamic equinus foot.
METHODS: Botulinum neurotoxin A was injected in the gastrocnemius, soleus and tibialis posterior muscles. The main measures were: pedobarometry, dynamic surface electromyography, video gait analysis scale, and the modified Ashworth Scale.
RESULTS: After treatment with botulinum neurotoxin A, pedobarometric evaluation revealed a significant change in all parameters, including a decrease in the modified Ashworth Scale and an increase in the range of motion. All children showed significant improvement in initial foot contact, as documented by the video gait analysis scale. The calf muscle electromyography pattern showed a decrease in co-contraction during gait in all children. These modifications were statistically significant for all parameters considered (p < 0.05).
CONCLUSION: This pilot study suggests that dynamic electromyography and pedobarometry are simple to use and provide useful data; this protocol could be preferable in young and uncooperative children in order to monitor rehabilitation treatments.
Key words: dynamic electromyography, cerebral palsy, botulinum toxin, spasticity, pedobarometry.
J Rehabil Med 2007; 39: 115–120
Correspondence address: Paolo Manganotti, Dipartimento di Scienze Neurologiche e della Visione, Sezione di Neurologia Riabilitativa, Policlinico G.B. Rossi- Via A. Scuro, IT-37134 Verona, Italy. E-mail: paolo.manganotti@univr.it
Submitted November 8, 2005; accepted May 17, 2006
Spastic equinus foot is the most common leg deformity in children with cerebral palsy (CP) who are able to ambulate (1). Several open-label studies have supported the effectiveness of botulinum toxin A (BTX-A) treatment for pes equinus in CP, demonstrating significant clinical and instrumental changes (2–6). In reality, patients who do not respond to BTX treatment have also been described, although they are a minority. The possibility to improve quantitative analysis of the therapeutic effects of botulinum toxin is thus an important field of clinical research.
The effectiveness of electromyographic (EMG) analysis of
gait as an important method to assist clinicians in the description of abnormal phasic muscle activity in children with CP has been demonstrated in several open studies (7, 8). The EMG pattern can add valuable information for differential diagnosis between mild diplegic CP and idiopathic toe-walking (9) in children and represents a useful feedback for the effectiveness of BTX-A treatment (5, 10, 11). Pedobarometry is another help-
ful clinical tool to investigate the postural effects following BTX-A injection in children with CP and pes equinus. Previous
studies have shown that this evaluation can be used in normal adult subjects (12, 13) and in patients affected by rheumatoid arthritis (14) or diabetic foot (15) in order to acquire data under
static conditions. In a previous study we demonstrated the effectiveness of pedobarometric evaluation for monitoring the effects of BTX-A injection on postural attitude (16). The objective of this pilot study was to develop a clinical and instrumental protocol to assess the postural and dynamic effects of treatment with BTX-A in children with CP affected by spastic equinus deformity, using pedobarometry and surface EMG.
METHODS
Participants
We evaluated 10 children (7 boys and 3 girls) with CP who exhibited signs of unilateral spastic equinus; all were community ambulators. Three presented mild impairment of the upper limb consequent to reduced control and none presented spasticity of the thigh or upper limb muscles. The mean age at the time of treatment was 9 years (standard deviation (SD) 2.39), with a range of 6–13 years. Sequential outpatients were recruited from different multidisciplinary clinics for treatment of children with CP, using the following inclusion criteria: ability to ambulate (assisted or unassisted), spastic hemiplegia or monoplegia, spastic equinus, no previous treatment with BTX-A or any anti-spastic drug, no fixed contractures and no previous surgeries of the foot, ankle and/or leg. All patients presented a score of 2 on the Gross Motor Function Scale.
We selected also a control group of 10 healthy children age-matched for speed comparison (mean age 9.2 years, SD 2.14; age range 6–14 years).
Informed consent was obtained from the children’s parents before beginning studies. We also received approval from the hospital ethics committee.
Evaluation protocol
At the first visit (T1), comprehensive clinical (ROM = range of motion, presence of clonus, Modified Ashworth Scale, video gait analysis (VGA)) and instrumental (pedobarography, EMG) examinations were performed; in the same session, all children received a BTX-A injection. One month later (T2), patients underwent a new examination with the same procedures used at T1.
Clinical examination
Clinical examination included a single passive range of motion (electronic goniometer) evaluation of the ankle of the affected side, clonus elicited by a rapid ankle dorsiflexion and grade of spasticity (modified Ashworth Scale) of the plantar flexor and invertor muscles. Children were examined by the same physician (MF) at time T1 (before BTX-A injection) and at time T2 (one month after BTX-A injection). One as-
sessment was performed at each visit.
Pedobarometric assessment
Pedobarometric evaluation was performed at the Clinical Hospital GB
Rossi of Verona. The pedobarometric equipment used includes a force
plate (FAS system 1.0 ACP Light), with an active surface (47.5 × 43.0 cm)
equipped with 2544 optical sensors, distributed along the perimetrical border. The pedobarometric static test was performed with children in a standing position with outstretched arms and looking at a fixed point for a period of 14 seconds. Two static tests were sequentially registered for each patient before and after BTX-A injection. The
mean value of the 2 evaluations of each quantitative pedobarometric data was considered at times T1 and T2. Quantitative analysis of pedobarometric evaluation included the entire plantar surface area (expressed in cm2), the peak pressure values at the forefoot and hindfoot (expressed in kilopascals), and the distance between the body centre of mass (COM) and the centre of pressure (COP) of each foot (expressed in cm).
Gait analysis
At each visit, video gait was recorded in both coronal and sagittal planes to establish the patient’s initial foot contact during the stance phase. Observational gait analysis was performed using VGA scoring, a form of observational gait analysis. On this scale, the physician
evaluated the initial foot contact during the stance phase, while patients walked barefoot over a distance of 12 metres. This scale (17) graded each moment of the stance phase as follows: normal heel strike (0); flat foot (1); toe then heel (2), mild toe walking (3); marked toe walking (4). A change of one grade, in either one or both treated legs, can be considered as clinically significant.
For the measurement of basic gait-cycle parameters, patients walked barefoot along a 10-metre walkway at maximum speed. The time needed for this was measured with a stopwatch, and the numbers of steps were counted. This enabled the calculation of velocity and cadence, and the mean stride length was then determined by dividing the gait velocity by cadence and multiplying it by 2 (18).
Dynamic video electromyography
The EMG signal was recorded using 5 mm electrodes coated with silver chloride. The tibialis anterior and gastrocnemius medialis were used for recording. Two electrodes were placed on each muscle from edge to edge of the electrode collars in the direction of the muscle fibres, midway between the proximal and distal ends of the superficially
palpable part of the muscle fibres. The skin was scratched lightly before positioning each electrode, reducing the impedance below 5000 Ohm. The sampling rate was 1024 Hz. The lower limb movements were recorded with a video camera at 60 frames per second, and a voltage pulse synchronizing each frame was recorded with the EMG. We used a video polygraphy EMG system (Micromed System Brainquick, Italy). For all subjects, video- EMG was recorded with children in a static condition and walking barefoot at a freely selected speed. This enabled dynamic EMG agonist/ antagonist muscle activity to be analysed in relation to the stance/swing phase of gait. The EMG data were digitally filtered (band-pass, 30–400 Hz), rectified, averaged over at least 10 strides and time-normalized. The duration and the area of the burst of activation of each muscle were measured on the averaged track.
Botulinum toxin intervention
Clinical and instrumental examination suggested which muscles required treatment. All injections were administered by 2 of the authors (PM, MF) under surface anaesthesia with ethyl chloride spray using standardized injection techniques under sterile conditions. Injection sites were identified using surface anatomy and an EMG guide to be as close as possible to motor endplates. Electrical stimulation then confirmed needle placement. BTX-A (Botox®, Allergan Inc, Irvine, CA, USA) reconstituted with normal
saline at a concentration of 100 U in 2 ml was used for all injections. A Tefloncoated Botox injection needle (37 mm, 27 gauge) was used, allowing electrical stimulation to confirm needle placement. Doses of Botox were administered according to the recommendations by Russman (19): 2–6 U of BTX-A/kg bodyweight for gastrocnemius (medial and lateral) and soleus, 1–2 U of BTX-A/kg bodyweight for tibialis posterior, while the total dose did not exceed 12 U/kg (or 400 U) per visit, and a maximum dose of 50 U (volume 1 ml) per injection site. Once the appropriate muscle was located with electrical stimulation, the needle was aspirated to ensure that the dose of BTX-A was not injected into the vascular compartment. All patients were discharged from the hospital after 30 min of observation. We observed no injection-related complications.
Physiotherapy
At the initial evaluation, all patients had been undergoing physiotherapy
for at least 6 months, 5 days a week, in 1-hour sessions consisting of postural control exercises, gait training, passive and active inferior limb kinesis. The same treatment was performed from the first day after BTXA injection until the control visit and lasted 2 hours per day.
Analysis of results
A descriptive statistical study of the quantitative parameters of mean (M) and SD was performed. The effect of BTX-A injections on all
pedobarometric and EMG variables was examined using one-way analysis of variance (ANOVA). To compare variables, a paired sample t-test was also used. A p < 0.05 was considered statistically significant.
RESULTS
Clinical outcome measures
Clinical examination performed before and one month after BTX-A treatment demonstrated a significant reduction in spasticity of plantar flexor and invertor muscles, a decrease in clonus and an increase in the passive dorsiflexion ankle range of motion (pROM) on the treated limb of all patients.
Pedobarometric evaluation
We observed significant differences between the affected (A) and unaffected (UA) lower limb in the static pedobarometric measurements of all children (Fig. 1).
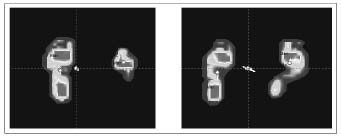
Fig. 1. Pedobarometric evaluation pre-treatment (left panel ) and post-treatment (right panel).
The main findings of our pedobarometric evaluation were (Table I):
| Table I. Pedobarometric parameters | ||||||
| Plantar surface area | PP hindfoot affected side | COM Shift | ||||
| Patients | First visit | After 1 month | First visit | After 1 month | First visit | After 1 month |
| 1 | 40.12 | 80.85 | 0 | 89.98 | 9.4 | 4.9 |
| 2 | 37.04 | 59.12 | 37.90 | 109.78 | 9.3 | 5.7 |
| 3 | 45.00 | 88.41 | 23.16 | 98.08 | 12.4 | 5.5 |
| 4 | 38.19 | 72.21 | 0 | 95.67 | 11.5 | 9.5 |
| 5 | 38.11 | 77.62 | 0 | 92.51 | 7.7 | 5.7 |
| 6 | 43.12 | 82.32 | 78.66 | 112.45 | 12.3 | 6.3 |
| 7 | 51.76 | 98.17 | 44.94 | 117.13 | 14.2 | 5.2 |
| 8 | 41.11 | 87.90 | 31.11 | 104.30 | 15.8 | 9.1 |
| 9 | 57.69 | 104.30 | 0 | 99.10 | 14.2 | 6.3 |
| 10 | 40.06 | 80.40 | 40.13 | 126.80 | 15.7 | 7.6 |
| Mean | 43.22 | 83.13 | 25.59 | 104.58 | 12.52 | 6.58 |
| Standard deviation | (6.65) | (12.75) | (26.26) | (11.78) | (2.79) | (1.62) |
| t -test | p < 0.05 | p < 0.05 | p < 0.05 | |||
| PP: peak of pressure; COM: centre of mass. | ||||||
• an increase in the whole plantar surface area (cm2) on the A side between time T1 and time T2 (43.22 vs 83.13) (p < 0.05);
• a significant increase in the peak pressure value (Kpa) on the hindfoot of the A side between time T1 and T2 (25.59 vs 104.58, p < 0.05);
• a significant shift of the body COM to the A side between time T1 and time T2, with a significant decrease of the distance (cm) between the body COM and the COP of the affected foot (12.25 vs 6.58, p < 0.05).
Furthermore, a significant decrease in the peak pressure value at the hindfoot was described on the UA side (108.9 vs 100.12, p < 0.05).
Video gait analysis
A significant improvement was observed in initial foot contact at 4 weeks (3.5 vs 1.5, p < 0.05) following administration of BTX-A in 8 children (Table II). However, significant increases in walking speed (metres/second) were not observed in all children (0.682 vs 0.672, p < 0.05); nonetheless, the speed of this group was not significantly different from a control group of 10 healthy children pair-aged (0.672 vs 0.704, p > 0.05).
| Table II. VGA and gait velocity | ||||
| Visual gait analysis (score) | Gait velocity (m/sec) | |||
| Patients | First visit | After 1 month | First visit | After 1 month |
| 1 | 4 | 2 | 0.72 | 0.69 |
| 2 | 3 | 2 | 0.58 | 0.58 |
| 3 | 3 | 1 | 0.63 | 0.62 |
| 4 | 4 | 1 | 0.67 | 0.68 |
| 5 | 4 | 1 | 0.78 | 0.80 |
| 6 | 3 | 1 | 0.69 | 0.69 |
| 7 | 3 | 1 | 0.76 | 0.73 |
| 8 | 3 | 0 | 0.80 | 0.81 |
| 9 | 4 | 3 | 0.54 | 0.48 |
| 10 | 4 | 3 | 0.65 | 0.64 |
| Mean | 3.5 | 1.5 | 0.682 | 0.672 |
| SD | (0.53) | (0.97) | (0.09) | (0.10) |
| t -test | p < 0.05 | p < 0.05 | ||
Dynamic electromyographic parameters
The calf muscle EMG pattern was improved by treatment in all children at time T2 (Figs. 2 and 3). The co-contraction of plantar flexor and dorsiflexor muscles of these children decreased significantly during the midstance of gait.
Fig. 2. Dynamic electromyographic raw data pre-treatment (left panel ) and post-treatment (right panel).
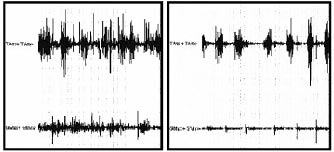
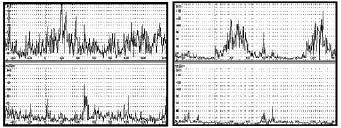
Fig. 3. Dynamic electromyographic average pre-treatment (left panel) and post-treatment (right panel).
The area (μV•ms) of EMG bursts of these 2 muscles decreased significantly (gastrocnemius medialis: 46563.2 vs 6747.6, p < 0.05; tibialis anterior: 22293.2 vs 9387.6, p < 0.05). The duration of these bursts of activation decreased significantly after the treatment: 792.7 vs 589.9 ms (p < 0.05) for the medial head of gastrocnemius muscle and 656.0 vs 467.9 ms (p < 0.05) for the tibialis anterior (Table III).
| Table III. EMG parameters | ||||||||
| EMG | ||||||||
| TA burst area | GM burst area | TA burst duration | GM burst duration | |||||
| Patients | First visit | After 1 month | First visit | After 1 month | First visit | After 1 month | First visit | After 1 month |
| 1 | 20098.7 | 11098.2 | 40911.4 | 5876.1 | 602.3 | 459.2 | 650.2 | 580.4 |
| 2 | 34987.5 | 7412.1 | 34289.7 | 8300.4 | 780.2 | 500.3 | 992.9 | 852.2 |
| 3 | 28450.0 | 7890.1 | 52175.8 | 11285.3 | 719.9 | 455.7 | 849.9 | 804.2 |
| 4 | 16887.2 | 8076.2 | 68142.2 | 9921.3 | 422.3 | 404.1 | 777.3 | 406.3 |
| 5 | 15222.1 | 13451.3 | 44671.3 | 6599.1 | 588.7 | 512.1 | 678.1 | 528.8 |
| 6 | 13980.7 | 9500.9 | 29641.2 | 4407.9 | 602.5 | 407.2 | 650.7 | 506.6 |
| 7 | 31491.6 | 14092.0 | 47891.4 | 5274.2 | 556.7 | 420.6 | 756.5 | 512.5 |
| 8 | 34640.2 | 7685.0 | 31772.1 | 5470.6 | 637.4 | 491.6 | 792.4 | 409.9 |
| 9 | 12837.2 | 7757.9 | 61003.4 | 6317.9 | 752.1 | 538.8 | 782.1 | 653.2 |
| 10 | 14336.8 | 6912.3 | 55133.5 | 4023.2 | 897.9 | 489.4 | 996.9 | 644.9 |
| Mean | 22293.2 | 9387.6 | 46563.2 | 6747.6 | 656.0 | 467.9 | 792.7 | 589.9 |
| SD | (9079.7) | (2606.2) | (12789.7) | (2374.4) | (134.3) | (46.27) | (124.9) | (150.8) |
| t-test | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | ||||
| TA: tibialis anterior muscle; GM: gastrocnemius medialis muscle. | ||||||||
DISCUSSION
The main finding of this study is that a decrease of hypertonia in foot flexor and invertor muscles on the treated limb can change the postural attitude and body stability of children with CP during standing and also modifies the gait disorder, as revealed by both pedobarometry and dynamic EMG. These tools are sensitive and useful for objective quantification of the clinical changes following treatment. This study confirms the efficacy of our protocol in evaluating the effects of BTX-A injection in treatment of children with CP and equinus.
Clinical data support the effectiveness of BTX-A injections for the treatment of CP considering the entity of impairment and functional limitation activity, as documented by several controlled studies (10, 11, 20–22). This improvement was clearly due to the pharmacological treatment (that is amplified by kinesis) and not just to physiotherapy, since 6 months of intensive kinesis did not lead to significant clinical changes in these patients. Reduction in muscle tone and gait improvement were evaluated by clinical scales and visual observation: we noted the utility of the Ashworth scale, the ROM evaluation and VGA score as tools to quantify clinical changes in children with CP treated with BTX-A. The modified Ashworth scale (MAS) was used because, even though it is not validated for ankle movement in children with CP, it seemed to be able to assess intra-subject changes.
Reducing the hypertonous of the plantar flexor and invertor
muscles, we observed an increase in the entire plantar surface area on the affected side that was not observed on the UA side
and a global improvement in all pedobarometric parameters. These significant changes show that such a tool can be used to
indirectly measure increases in body stability and modifications of postural attitude in children; parameters that cannot be assessed appropriately in dynamic conditions. While patients were able to achieve better stability, there was no change in walking speed. While the former is an important safety issue in adults, it can be translated into a better basis for maintaining ambulation into later life in children with CP.
We believe that evaluation of functional outcome following BTX-A treatment in CP cannot exclude the investigation of postural changes in static conditions, since dynamic evaluation cannot reveal these clinical features. One of the main aims of the present study was to standardize static pedobarometric evaluation in children with CP, using the same procedure as proposed in adults (12). Furthermore, using this approach we avoided the training effect by performing 2 single trials for each child during each evaluation. We did not perform more than 2 trials. In addition, visual feedback was avoided in all patients who were obliged to look at a fixed point and not at the video.
In children with CP and equinovarus foot deformity, dynamic EMG recordings showed that the leg muscle activation pattern is altered during gait. Plantarflexors often co-activate with overpowering of the tibialis posterior and gastrocnemius during the swing phase, producing initial ground contact with the toe, rather than the heel (23). This data has been described as a consequence of an abnormal velocity-dependent EMG recruitment during muscle stretch (spastic component) and of a nonselective activation of agonist-antagonist muscles with loss of the normal reciprocal inhibitory pattern (co-contraction component) (24). The findings of our dynamic EMG analysis showed a decrease in the co-contraction pattern of the anterior tibial and triceps surae muscles during the swing of gait in all children. The improvement of muscular pattern is difficult to explain simply by an effect of BTX-A at the neuromuscular junction. Several studies have described possible mechanisms to explain the functional effect of BTX-A in treatment of lower limb hypertonous in children with CP. Therefore, the improvement in reciprocal inhibition following BTX-A injection
could explain a decrease in the duration of co-activation of lower limb agonist-antagonist muscles, thereby improving the quality of gait in these children (11), but also the improvement in biomechanics of initial contact can be effective in changing the pattern (25). Speed, as documented by some authors, can also influence the EMG pattern; this was not the case since gait velocity did not change in a homogeneous manner, while muscular activation improved in all patients.
In routine clinical practice, such an investigational method (pedobarometry and EMG) that is easy and quick to carry out may be useful when children are either too young or are too uncooperative for instrumented gait analysis (26). Importantly, the capability objectively to assess the effect of a treatment is particularly relevant especially when the given treatment is invasive like BTX-A, and when the expected improvement is not so clinically evident.
In conclusion, this pilot study suggests that the effect of BTX-A treatment of dynamic equinus foot deformity in children
with CP can be monitored with dynamic EMG and pedobarometry in order to collect more objective data that integrate and complete clinical evidence. Nonetheless, further studies are needed to confirm these preliminary results collected on a small population and without a control group for most parameters, in order to extend the use of this clinical and instrumental pproach to different groups of children with CP.
REFERENCES
1. Banks HH. The management of spastic deformities of the foot and ankle. Clin Orthop 1977; 122: 70–76.
2. Burbaud P, Wiart L, Dubos JL, Gaujard E, Debelleix X, Joseph PA, et al. A randomized, double-blind, placebo-controlled trial of botulinum toxin in the treatment of spastic foot in hemiparetic patients. J Neurol Neurosurg Psychiatry 1996; 61: 265–269.
3. Hesse S, Luecke D, Malezic M, Bertelt C, Friedrich H, Gregoric M, et al. Botulinum toxin treatment for lower limb extensor spasticity in chronic hemiparetic patients. J Neurol Neurosurg Psychiatry 1994; 57: 1321–1324.
4. Zuercher AW, Molenaers G, Desloovere K, Fabry G. Kinematic and kinetic evaluation of the ankle after intramuscular injection of botulinum toxin A in children with cerebral palsy. Acta Orthop Belg 2001; 67: 475–480.
5. Metaxiotis D, Siebel A, Doederlein L. Repeated botulinum toxin A injections in the treatment of spastic equinus foot. Clin Orthop Relat Res 2002; 394: 177–185.
6. Polak F, Morton R, WardWA, Doderlein L, Siebel A. Double-blind comparison study of two doses of botulinum toxin A injected into calf muscles in children with hemiplegic cerebral palsy. Dev Med Child Neurol 2002; 44: 551–555.
7. Dimitrijevic MR, Faganel J, Sherwood AM, McKayWB. Activation of paralysed leg flexors and extensors during gait in patients after stroke. Scand J Rehabil Med 1981; 13: 109–115.
8. Perry J, Hoffer MM, Giovan P, Antonelli D, Greenberg R. Gait analysis of the triceps surae in cerebral palsy. A preoperative and postoperative clinical and electromyographic study. J Bone Joint Surg Am 1974; 56: 511–520.
9. Davids JR, Foti T, Dabelstein J, Bagley A. Voluntary (normal) versus obligatory (cerebral palsy) toe-walking in children: a kinematic, kinetic, and electromyographic analysis. J Pediatr Orthop 1999; 19: 461–469.
10. Sutherland DH, Kaufman KR, Wyatt MP. Double-blind study of botulinum A toxin injections into the gastrocnemius muscle in patients with cerebral palsy: a 3-dimensional motion analysis study. Gait Posture 1996; 10: 269–279.
11. Corry IS, Cosgrove AP, Duffy CM. Botulinum toxin A in hamstring spasticity. Gait Posture 1999; 10: 206–210.
12. Imamura M, Imamura ST, Salomao O, Pereira CA, De Carvalho AE Jr, Neto RB. Pedobarometric evaluation of the normal adult male foot. Foot Ankle Int 2002; 23: 804–810.
13. Hughes J, Clark P, Linge K, Klenerman L. A comparison of two studies of the pressure distribution under the feet of normal subjects using different equipment. Foot Ankle 1993; 14: 514–519.
14. Minns RJ, Craxford AD. Pressure under the forefoot in rheumatoid arthritis. A comparison of static and dynamic methods of assessment. Clin Orthop 1984; 187: 235–242.
15. Sarnow MR, Veves A, Giurini JM, Rosenblum BI, Chrzan JS, Habershaw GM. In-shoe foot pressure measurements in diabetic patients with at-risk feet and healthy subjects. Diabetes Care 1994; 17: 1002–1006.
16. Falso M, Fiaschi A, Manganotti P. Pedobarometric evaluation of equinus foot disorder after injection of botulinum toxin A in children with cerebral palsy: a pilot study. Dev Med Child Neurol 2005; 47: 396–402.
17. Lin JP, Brown JK. Peripheral and central mechanisms of hindfoot equinus in childhood hemiplegia. Dev Med Child Neurol 1992; 34: 949–965.
18. Hesse S, Krajnik J, Luecke D, Jahnke MT, Gregoric M, Mauritz KH. Ankle muscle activity before and after botulinum toxin therapy for lower limb extensor spasticity in chronic hemiparetic patients. Stroke 1996; 27: 455–460.
19. Russman BS. Cerebral Palsy. Curr Treat Options Neurol 2000; 2: 97–108.
20. Barwood S, Baillieu C, Boyd R. Analgesic effects of botulinum toxin A: a randomized, placebo-controlled clinical trial. Dev Med Child Neurol 2000; 42: 116–121.
21. Cosgrove AP, Corry IS, Graham HK. Botulinum toxin in the management of the lower limb in cerebral palsy. Dev Med Child Neurol 1994; 36: 386–396.
22. Kirschner J, Berweck S, Mall V, Korinthenberg R, Heinen F. Botulinum toxin treatment in cerebral palsy: evidence for a new treatment option. J Neurol 2001; 248: 28–30.
23. Colborne GR, Wright FV, Naumann S. Feedback of triceps surae EMG in gait of children with cerebral palsy: a controlled study. Arch Phys Med Rehabil 1994; 75: 40–45.
24. Crenna P, Cuong DM, Breniere Y. Motor programmes for the termination of gait in humans: organisation and velocity-dependent adaptation. J Physiol 2001; 537: 1059–1072.
25. Hesse S, Brandl-Hesse B, Seidel U, Doll B, Gregoric M. Lower limb muscle activity in ambulatory children with cerebral palsy before and after the treatment with Botulinum toxin A. Restor Neurol Neurosci 2000; 17: 1–8.
26. Koman LA, Mooney III JF, Smith BP. Botulinum toxin type A neuromuscular blockade in the treatment of lower extremity spasticity in cerebral palsy: a randomized, double-blind, placebocontrolled trial. Botox Study Group. J Pediatr Orthop 2000; 20: 108–115.
