OBJECTIVE: To evaluate changes over time in a clinically based cohort of individuals with post-polio syndrome.
DESIGN: A prospective longitudinal study.
SUBJECTS: A total of 106 individuals with poliomyelitis sequelae were included in the study. They were self-referred or had been referred to the post-polio clinic. After 4 years subjects were called for a follow-up and underwent the same measurements as at the initial assessment.
METHODS: The following measurements were conducted at both the initial assessment, and the follow-up: questionnaires including Nottingham Health Profile, muscle strength and walking speed.
RESULTS: Minor changes in disability during a 4-year period were shown. A significant reduction in muscle strength was only seen for 60° flexion in the left leg and for right and left dorsal flexion. No change could be seen in the total Nottingham Health Profile score.
CONCLUSION: The minor changes in disability found in this study are an indication that we still do not know which subjects are at risk for deterioration. It is difficult to say whether the small changes over time shown in this study are associated with support from the polio clinic or are an expression of the natural history of the syndrome. However, it is hoped that support from the polio clinic may result in self-selected lifestyle changes, which may positively influence the development of symptoms and functional capacity.
Key words: post-polio, muscle strength measurements, walking test, health-related quality of life.
J Rehabil Med 2007; 39: 175–180
Correspondence address: Carin Willén, Institute of Neurosciences and Physiology, Guldhedsgatan 19, SE-413 45 Göteborg, Sweden. E-mail: carin.willen@fhs.gu.se
Submitted April 4, 2006; accepted October 11, 2006
*This article has been fully handled by one of the Associate Editors, who has made the decision for acceptance, as it originates from the institute where the Editor-in-Chief being a coauthor is active.
INTRODUCTION
For a long time, poliomyelitis was a major health problem. The disease has disappeared as a major public health risk in western countries, but new health problems have been reported in individuals with a history of acute poliomyelitis (1, 2). These new problems, which are related to the previous disease and occur more than 15 years after the acute illness, include fatigue, new or increased muscle weakness and muscle and joint pain (3–7). The impact of these problems on the patient’s activity level is mainly on mobility, and on physical demands in the home or at work (8, 9). The most widely known term used to describe these new symptoms is post-polio-syndrome (PPS) (10). The criteria for diagnosing PPS have recently been revised (11) (Table I).
In a 4-year cohort study of Swedish and American individuals post-polio, a significant reduction in knee extensor strength (8%) was found (7). There was no difference between those acknowledging PPS and those who did not. An 8-year follow-up study by Grimby et al. (12) showed evidence of a progressive reduction in muscle strength in the knee extensors over the study period (9–15%). A more pronounced reduction in muscle strength was seen in the unstable legs (increased symptomatic muscle weakness) than in the stable ones (no new or increased muscle weakness), but there was a large variation in muscle strength in both groups. Findings contradictory to these were made by Windebank et al. (13), who reported that no progress was seen over a 5-year period. In a recent review it was suggested that a period of about 4 years was a minimum to detect a decline in strength (14).
A few other studies have also focused on both functioning and perceived health (15, 16). In a 5-year follow-up study of 63 patients with PPS, physical disability related to the polio disease was found to increase with age, and cardiorespiratory deconditioning and weight gain were regarded as increasing problems (15). However, the patients’ mental status remained stable or improved. In a study by Nollet et al. (16) a cohort of 103 polio survivors, 76 diagnosed as having PPS and 27 as not having it, were studied prospectively for 6 years in terms of health status in general and physical function in particular. In overall terms there was little or no change in perceived health status over time in the subjects with or without PPS.
However, to understand the progression of PPS and its consequences, more longitudinal studies are needed. The aim of the present study was prospectively to evaluate changes over time in disability in a clinically based cohort of individuals with PPS.
METHODS
Subjects
A total of 106 individuals with poliomyelitis sequelae were included in the study. They were self-referred or had been referred to the post-polio clinic at Sahlgrenska University Hospital, Göteborg, which aims to offer services to individuals with an early history of polio. After 4 years subjects were called for a follow-up and the individuals underwent the measurements described below, once again.
The post-polio clinic was organized as a multidisciplinary team, including a physician, a nurse, an occupational therapist, a physical therapist, a social worker and a secretary.
On entering the clinic the individual underwent basic laboratory examinations and was then seen by the nurse, the physician, the occupational therapist and the physical therapist, each of whom performed specific assessments. If necessary an appointment with the social worker was organized. After the individual had been seen by the team members a team conference was held. At this conference recommendations for interventions were made. A treatment plan was discussed and, if necessary, referrals to other healthcare providers were made. For some individuals physical therapy, such as aquatic therapy, could be provided at the clinic.
The study was approved by the Ethics Committee at the Faculty of Medicine, Göteborg University. All the subjects were informed about the study and gave their verbal consent to participate in a 4-year follow-up.
Measurement instruments
The following measurements were conducted at both the first assessment and the follow-up:
Questionnaires. A questionnaire was posted to the subjects before the first appointment. It contained questions about patient demographics, age at acute polio onset, body part(s) affected, onset of new or increasing health problems related to the polio disease, other diseases and the current use of walking devices. If necessary, the questionnaire was completed during the visit to the physician. The same procedure was repeated at the follow-up.
The Swedish version of the Nottingham Health Profile (NHP) was used to describe physical, emotional and social aspects of perceived health (17). The instrument consists of 2 parts, although only data from part one is presented in the present study. It has 6 dimensions assessing distress in emotional reaction, sleep, energy, pain, physical mobility and social isolation. The individual answer yes or no to 38 different statements and each answer is multiplied by a specific weight and the weighted mean for each dimension is calculated. The higher the score the higher the level of perceived distress in one dimension, the maximum score being 100.
Muscle strength. Muscle strength was measured using an isokinetic dynamometer (KINetic-COMmunicator, Chattex, Chattanooga, USA). For measurements of the knee extensors and knee flexors, the subject was seated with his or her back against the seat. A seat-belt was strapped around the waist and thigh to avoid unwanted movements. Prior to the test the subject warmed up for 5 minutes on a bicycle ergometer. Peak isometric strength was measured at a 60° knee angle in extension and flexion. Maximal isokinetic strength was measured at a velocity of 60°/s. For measurements of calf muscle strength, the subjects were in a supine position, secured to the bench with a belt around their waists. The hip and knee were straight with the tested foot outside the bench. The isometric strength in Nm of foot dorsal and plantar flexors at 0° was measured. All the measurements were performed on both legs if possible and the test values were compared with and expressed as a percentage of control values, corrected for age and sex, from a random population (18).
Walking. Spontaneously and maximal chosen walking speeds were measured as the time taken to walk 30 metres indoors. The test started with the spontaneously chosen speed. The subjects used their own footwear and walking devices if necessary.
Electromyography (EMG) (conducted only at the initial assessment). The EMG examination, together with the muscle strength measurements, was conducted at the laboratory after the visit to the clinic. The presence of polio-affected muscles was first determined according to the clinical history of the subject, the clinical findings and together with the EMG results from at least 3–5 relevant muscle groups according to the individual’s polio history. The diagnosis of PPS was determined according to the criteria outlined by Gawne & Halstead (10) (Table I).
| Table I. Criteria for the diagnosis of post-polio syndrome |
| • Prior episode of paralytic polio with residual motor neurone loss (which can be confirmed through a typical patient history, a neurological examination and, if needed, an EMG examination). |
| • A period of neurological recovery followed by an interval (usually ≥ 15 years) of neurological and functional stability. |
| • Gradual or abrupt onset of progressive new weakness with a duration of at least 12 months, or abnormal muscle fatigability, with or without generalized fatigue, muscle atrophy, or pain. |
| • Exclusion of medical, orthopaedic, or neurological conditions that may be causing the symptoms mentioned above. |
| EMG: electromyography. |
When all the examinations had been completed the individual was scheduled for a follow-up appointment with the physician, where the test results were gone over and a plan for the future was discussed. Further follow-up appointments were scheduled according to the individual’s need.
Statistics
Conventional formulae were used to calculate the mean, median, standard deviations, range percentiles and confidence intervals. For tests of paired differences in continuous variables the paired t-test was used, and the Sign test was used to test differences in dicotomy data. For tests of several independent samples the Kruskal-Wallis test was used, while the Mann-Whitney non-parametric U test was used for tests of 2 independent samples. The association between change in muscle strength and muscle strength at the initial assessment was analysed with linear regression. The variable age was included in the model. A secondary analysis was conducted to assess whether the group of 9 subjects with stable muscle function could be distinguished from the total group. A significance value of p < 0.05 was used throughout the study.
RESULTS
Of the 106 subjects, 97 were diagnosed as having PPS at the first assessment (Table II). The remaining 9 reported a stable muscle function (no new or increased muscle weakness). Six of them developed PPS during the 4-year period. The secondary analysis showed that the 9 individuals with non-PPS could not be distinguished from the total group in terms of anything other than age, as the stable group was younger than the unstable group (p < 0.04). As a result, all 106 were treated as one group in the further analyses.
| Table II. Characteristics of the individuals and the number of persons with or without post-polio syndrome (PPS) at the initial assessment |
| Characteristics | Men (n = 44) | Women (n = 62) |
| Age (years) |
| < 45 | 9 | 12 |
| 45–64 | 30 | 29 |
| > 64 | 5 | 21 |
| Mean age at onset of disease (years) (range) | 7 (0–31) | 6 (0–28) |
| Stable phase (years) (range) | 33 (7–50) | 33 (10–60) |
| PPS | 39 | 58 |
| Non-PPS | 5 | 4 |
The subjective experiences of new health problems relating to fatigue, new weakness in muscles not previously affected, and pain, showed no change at the follow-up compared with the first assessment (Table III). There was, however, a significant difference in difficulties experienced in mobility activities, such as walking on the ground and up and down stairs (p < 0.000 for both the activities), creating more problems for the individual at the follow-up. The number of subjects using walking devices had increased over the 4 years. For example, there was an increase in the use of wheeled walking frames from 5% to 20%. The use of foot orthoses had increased from 10% to 22% in the group, and at the follow-up assessment shoe inserts were used by over half of the group, 52% compared with 37% at the initial assessment.
| Table III. Percentage of persons who reported subjective experiences of health problems at the initial and follow-up assessments (n = 106) |
| Health problem | Initial (%) | Follow-up (%) |
| General fatigue | 82 | 78 |
| New weakness in muscles with earlier weakness | 81 | 81 |
| New weakness in muscles with no earlier weakness | 43 | 42 |
| Muscle pain at rest | 50 | 58 |
| Muscle pain during activity | 79 | 83 |
| Joint pain at rest | 40 | 48 |
| Joint pain during activity | 64 | 60 |
| Back pain | 64 | 60 |
| Breathing difficulties, rest | 14 | 8 |
| Breathing difficulties, exertion | 26 | 30 |
| Cold intolerance | 62 | 67 |
| Difficulty in walking on level surface* Yes Yes increased Cannot | 66 8 2 | 23 55 5 |
| Difficulty in walking up and down stairs* Yes Yes increased Cannot | 79 5 4 | 23 55 10 |
| *p < 0.001. |
Generally, there were small changes in the muscle strength measurements. A significant reduction in muscle strength was seen for 60° flexion in the left leg (p = 0.020) and dorsal flexion in both the right and left leg (p= 0.001 and p = 0.006 respectively) (Table IV). From the linear regression a significant association between the change in muscle strength and muscle strength at the initial assessment was seen for left and right knee flexion 60°/s, left and right dorsal flexion and left plantar flexion, indicating a greater decrease in change in strength if stronger at the initial assessment.
The difference in the body mass index (BMI) value was significant and the value had increased at the follow-up (p < 0.001) (Table IV). The average increase in weight was 2 kg for both men and women.
| Table IV. Change in body mass index (BMI), walking velocities and muscle strength measurements between initial assessment and follow-up. The tables include lower and upper 95% confidence limits (CL) |
| | n | Initial assessment | Mean difference | Lower CL | Upper CL | p-value |
| BMI | 89 | 24.76 | –0.7760 | –1.1017 | –0.4505 | 0.001 |
| Spontaneous walking (m/s) | 76 | 0.99 | 0.0925 | 0.0586 | 0.1264 | 0.001 |
| Maximal walking (m/s) | 76 | 1.20 | 0.0624 | 0.0155 | 0.1094 | 0.010 |
| Strength (Nm) (% of normal values) |
| Extension 60° right leg | 77 | 45.60 | 3.052 | –0.200 | 6.04 | 0,065 |
| Extension 60° left leg | 74 | 55.15 | 2.838 | –0.899 | 6.574 | 0.134 |
| Extension 60°/s right leg | 75 | 47.51 | 0.920 | –2.436 | 4.276 | 0.587 |
| Extension 60°/s left leg | 71 | 57.69 | 1.507 | –2.514 | 5.528 | 0.457 |
| Flexion 60° right leg | 76 | 57.61 | 2.250 | –1.408 | 5.908 | 0.224 |
| Flexion 60° left leg | 73 | 57.97 | 5.575 | 0.916 | 10.234 | 0.020 |
| Flexion 60°/s right leg | 76 | 58.74 | 0.605 | –4.523 | 5.733 | 0.815 |
| Flexion 60°/s left leg | 70 | 58.16 | 1.114 | –3.497 | 5.726 | 0.631 |
| Dorsal flexion right | 50 | 54.68 | 10.600 | 4.285 | 16.915 | 0.001 |
| Dorsal flexion left | 48 | 58.46 | 11.875 | 3.606 | 20.144 | 0.006 |
| Plantar flexion right | 40 | 49.53 | –1.600 | –6.949 | 3.749 | 0.495 |
| Plantar flexion left | 40 | 55.85 | –4.475 | –10.973 | 2.023 | 0.172 |
| Nm: Newton metres. |
In the walking tests a reduction in speed was seen in spontaneous walking and in maximal walking (p < 0.001 and p < 0.010, respectively) (Table IV). The mean decrease in spontaneous walking was 0.09 metres/second (m/s), while it was 0.06 m/s for maximal walking.
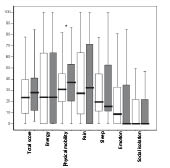
The NHP was analysed both for the whole group and then divided into 3 different age groups; < 45 years, 45–64 years and > 64 years. No change could be seen in the total NHP score for the whole group (Fig. 1). However, there was a significant deterioration in the dimension of physical mobility (p < 0.001), to which the oldest group, > 64 years, contributed. When analysing NHP between the 3 age groups a difference could be seen at the initial assessment where the youngest group, < 45 years, showed more distress in the 2 dimensions of pain and emotion in comparison with the oldest group (p < 0.009 and p < 0.007, respectively). These differences could still be seen after 4 years (p < 0.005 for pain and p < 0.003 for emotion).
Fig. 1. Median, 25th and 75th percentiles for the values in Nottingham Health Profile (NHP) at initial assessment ( ) and at follow-up ( ). *p < 0.001.
DISCUSSION
The results in the present follow-up study showed minor changes in disability during a 4-year period. The prevalence of symptoms was in accordance with other clinically based studies (19, 20, 4). The studied cohort was clinically based and a different result might have been found in a population-based study of polio survivors. It may be assumed that people with actual problems are more disposed to contact the healthcare system. Those individuals who were admitted to the post-polio clinic were perhaps initially more severely affected by polio and there might be less scope for deterioration in these subjects. However, in the study by Nollet et al. (16), there was no difference in the health status of individuals who had or did not have PPS. It was not surprising that the 9 individuals who did not fulfil the criteria for PPS at the first assessment were younger compared with the total group. The time since the onset of the disease was perhaps not long enough, for the development of PPS.
The reduction in walking speed was statistically significant, but the size of the change was minor (6–8%).Walking speed has been found to be a good predictor of independent mobility (21, 22). Hence, a retained walking speed must be regarded as positive for the individual in order to maintain independence. The individuals experienced more difficulties in walking on the ground and up and down stairs. This is supported by the study by Herwin et al. (23), who showed that in ambulant patients with PPS, walking in daily life were more demanding than walking in a test situation.
We could demonstrate a significant change in 1 of 4 measurements of knee flexion and in dorsal flexion in both legs. Grimby et al. (24) also showed in an earlier study a significant decrease in knee flexion after 4 years in the group of stable subjects (non-PPS) and also in extension in the unstable subjects. Prior longitudinal studies have assessed muscle strength in one leg as “the worst”; however, the muscle strength in those studies was in general higher than in the present group of individuals, which could be a factor explaining the different findings. It is not known whether the lack of demonstrated reduction in muscle strength in our study corresponds to stable compensatory mechanisms (re-innervation and muscle fibre hypertrophy) (25). This can also be caused by altered relative contribution of these mechanisms or reflect that a deterioration in function could be influenced and/or delayed by a change in lifestyle with less physical activity. This might be a result of the studied group being encouraged by the polio team. On the other hand, the reduced strength in the dorsal flexors could be explained by overuse, being particularly evident in that muscle group (26). The fact that those with stronger dorsal flexors at the initial assessment seemed to have a greater change in strength may reflect an effect of higher activity in those muscles. It is noteworthy that in the present study we examined both legs and both arms. As we also performed EMG on 3–4 extremities, we are aware that the effect of polio is more widespread than reported in the patients’ histories.
The reduced strength was, to a less extent, reflected in change in the walking speed at the examination. In a previous study, however, walking speed was not found to be associated with strength in single muscle groups (27). A combined strength index from various muscle groups involved in walking was therefore developed. It was shown that there was a non-linear relationship between the walking speed and the strength index (27). In the present study the reduction in muscle strength in the dorsal flexors was probably not great enough to influence the strength index sufficiently to influence the walking speed to a greater extent. After 4 years there was an increase in the prevalence of using of walking devices which might be a result of the visit at the polio clinic where devices were suggested and offered. This might be reflected in an increase in the walking speed. However, in another study we have shown that the use of walking devices does not increase walking speed, but is more likely used to compensate for other reasons, such as pain, risk of falling and fatigue (27).
There was a significant increase in BMI over the 4-year period, but the increase was not greater than that in the total Swedish population (28). It was, however, greater than in the Swedish sample in an American-Swedish study (7). Weight gain is a problem among individuals with polio, indicating that a greater increase in BMI could have been expected, as was also shown in the study by Stanghelle & Festvåg (15).
The health problems after 4 years showed little change for the NHP. This is in accordance with the study by Nollet et al. (16). When looking at changes in the NHP in the different age groups, the oldest group (≥ 65 years) contributed to the deterioration in the dimension of physical mobility. This is perhaps not surprising, as increasing age may increase the risk of developing symptoms from other diseases apart from polio, such as osteoarthritis and heart diseases, besides normal ageing, which also influences physical capacity. The differences between the age groups in the dimensions pain and emotion remained after 4 years. We know from earlier studies that the experience of pain may be related to the level of physical activity (29). Younger individuals with PPS are probably more active, as they have a different social life, including a higher degree of employment. Emotional distress was also more evident in the youngest group at the first assessment and at the follow-up. This difference was also seen in an earlier study by Thorén-Jönsson et al. (30). According to these authors, this could be explained by the fact that subjects at these ages are in a stage of life when disabilities increase, the life course is disrupted and continuing to realize earlier goals and expectations is difficult. Evidently, the period of 4 years was too short to alter this situation.
The minor changes in disability found in this study are an indication that we still do not know which subjects are at risk for deterioration. Because of the complexity of the problems listed by individuals with PPS, it has been suggested that interdisciplinary rehabilitation teams should be set up (31). A team of this kind supported the individuals in this study. It is difficult to say whether the small changes in disability over time shown in this study are associated with support from the polio clinic or are an expression of the natural history of the syndrome. However, support from others, as from the polio clinic, can result in one’s thoughts, experiences and problems being made legitimate and understood by others, and, with this, the process of accepting one’s situation and the will to adapt to it begins (32). Self-selected lifestyle changes may have positively influenced the development of symptoms and functional capacity.
ACKNOWLEDGEMENTS
This study was supported by grants from the Norrbacka Eugenia Foundation, the Hjalmar Svensson Research Foundation, the Swedish Association for Traffic and Polio Victims (RTP), the Gun and Bertil Stohne Foundation, the Olle Höök Foundation and the Axel Linder Foundation.
We wish to thank all the members of the Department of Rehabilitation Medicine, who participated in the data collection.
REFERENCES
1. Halstead LS, Wiechers DO, Rossi CD. Late effects of poliomyelitis: a national survey. In Halstead LS, Wiechers DO, editors. Late effects of poliomyelitis. Miami FL: Symposia Foundation; 1985, p. 11–13.
2. Westbrook M. A survey of post-poliomyelitis sequelae: manifestations, effects on peoples lives and responses to treatment. Aust Physiotherapy 1991; 37: 89–102.
3. Halstead LS, Rossi CD. New problems in old polio patients: results of a survey of 539 polio survivors. Orthopedics 1985: 7: 845–850.
4. Agre JC, Rodriquez AA, Sperling KB. Symptoms and clinical impressions of patients seen in a postpolio clinic. Arch Phys Med Rehabil 1989; 5: 367–370.
5. Lonnberg F. Late onset of polio sequelae in Denmark. Results of a nationwide survey of 3 607 polio survivors. Scand J Rehabil Med 1993; Suppl 28: 1–32.
6. Wekre LL, Stanghelle JK, Lobben B, Oyhagen S. The Norwegian polio study 1994: a nation-wide survey of problems in long-standing poliomyelitis. Spinal Cord 1998; 36: 280–284.
7. Agre JC, Grimby G, Rodriquez AA, Einarsson G, Swiggum ER, Franke TM. A comparison of symptoms between Swedish and American post-polio individuals and assessment of lower limb strength a four-year cohort study. Scand J Rehabil Med 1995; 3: 183–192.
8. Einarsson G, Grimby G. Disability and handicap in late poliomyelitis. Scand J Rehabil Med 1990; 22: 113–121.
9. Grimby G, Thorén Jönsson A-L. Disability in poliomyelitis sequelae. Phys Ther 1994; 74, 46–55.
10. Gawne CA, Halstead LS. Post-polio syndrome: pathophysiology and clinical management. Crit Rev Phys Rehabil Med 1995; 7: 147–188.
11. Halstead LS. Diagnosing postpolio syndrome:Inclusion and exclusion criteria. In: Silver JK, Gawne AC, editors. Postpolio syndrome. Philadelphia: Hanley and Belfus; 2004.
12. Grimby G, Stålberg E, Sandberg A, Stibrant Sunnerhagen K. An 8-year longitudinal study of muscle strength, muscle fiber size, and dynamic electrmyogram in individuals with late polio. Muscle Nerve 1998; 21: 1428–1437.
13. Windebank A, Litchy W, Daube JR, Iverson RA. Lack of progression in neurological deficit in survivors of paralytic polio. A 5-year prospective population-based study. Neurology 1996; 46: 80–84.
14. Stolwijk-Swüste J, Beelen A, Lankhorst J, Nollet F. The course of functional status and muscle strength in patients with late-onset sequelae of poliomyelitis: a systematic review. Arch Phys Med Rehabil 2005; 86: 1693–1701.
15. Stanghelle JK, Festvåg LV. Postpolio syndrome: a 5-year follow-up. Spinal Cord 1997; 35: 503–508.
16. Nollet F, Beelen A, Twisk J, Lankorst G, de Visser M. Perceived health and physical functioning in postpoliomyelitis syndrome: a 6-year prospective follow-up study. Arch Phys Med Rehabil 2003; 84: 1048–1056.
17. Wiklund I. The Nottingham Health Profile: a measure of health-related quality of life. Scand J Prim Health Care 1990; 1: 15–18.
18. Stibrant Sunnerhagen K, Hedberg M, Henning G-B, Cider Å, Svantesson U. Muscle performance in an urban population sample of 40- to 79-year-old men and women. Scand J Rehabil Med 2000; 4: 159–167.
19. Codd MP, Mulder DW, Kurland LT, Beard CM, O’Fallon W. Poliomyelitis in Rochester, MN 1935 to 1955. Epidemiology and long-term sequelae: a preliminary report. In: Halstead LS, Weichers DO, editors. Late effects of polio. Miami: Symposium Foundation Miami, FL; 1985, p. 121–133.
20. Cosgrove JL. Late effects of poliomyelitis. Arch Phys Med Rehabil 1987; 68: 4–7.
21. Sonn U, Frandin K, Grimby G. Instrumental activities of daily living related to impairments and functional limitations in 70-year-olds and changes between 70 and 76 years of age. Scand J Rehabil Med 1995; 2: 119–128.
22. Thorén-Jönsson A-L, Grimby G. Ability and perceived difficulty in daily activities in people with poliomyelitis sequelae. J Rehabil Med 2001; 1: 4–11.
23. Herwin H, Bussman J, Beelen A, Stam H, Nollet F. Walking in postpoliomyelitis syndrome: the relationships between time-scored tests, walking in daily life and perceived mobility problems. J Rehabil Med 2005; 37:142–146.
24. Grimby G, Hedberg M, Henning G-B. Changes in muscle morphology, strength and enzymes in a four-five-year follow-up of post-polio subjects. Scand J Rehabil Med 1994; 27: 121–130.
25. Einarsson G, Grimby G, Stålberg E. Electromyographic and morphological functional compensation in late poliomyelitis. Muscle Nerve 1990; 13: 165–171.
26. Grimby L, Tollbäck A, Muller U, Larsson L. Fatigue of chronically overused motor units in prior polio patients. Muscle Nerve 1996; 19: 728–737.
27. Willén C, Stibrant Sunnerhagen K, Grimby G. How is walking speed related to muscle strength in healthy individuals and subjects with late polio. Arch Phys Med Rehabil 2004; 18: 1923–1928.
28. Statistics. [Cited 2005 September 29]. Available from:
http://www.scb.se/templates/tableOrChart 47936.asp
29. Willén C, Grimby G. Pain, physical activity and disability in individuals with late effects of polio. Arch Phys Med Rehabil 1998: 79: 915–919.
30. Thorén-Jönsson A-L, Hedberg M, Grimby G. Distress in everyday life in people with poliomyelitis sequelae. J Rehabil Med 2001; 3: 119–127.
31. Gawne A. The interdisciplinary team assessment. In: Silver JK, Gawne AC, editors. Postpolio syndrome. Philadelphia: Hanley & Belfus; 2004.
32. Thorén-Jönsson A-L. Adaptation and ability in daily occupations in people with poliomyelitis sequelae. Doctoral thesis. University of Göteborg: Göteborg, Sweden; 2000.