William G. Tsiaras and Martin A. Weinstock
Dermatoepidemiology Unit, VA Medical Center, the Department of Dermatology, Rhode Island Hospital, and the Departments of Dermatology and Community Health, Brown University, Providence, Rhode Island
William G. Tsiaras and Martin A. Weinstock
Dermatoepidemiology Unit, VA Medical Center, the Department of Dermatology, Rhode Island Hospital, and the Departments of Dermatology and Community Health, Brown University, Providence, Rhode Island
The steroid hormone vitamin D is required for normal calcium and phosphorus metabolism and is thus an important contributor to musculoskeletal health. Recent data have linked low vitamin D levels to a wide range of diseases, including cancer, cardiovascular disease, autoimmune disease and infection. Adequate levels of vitamin D are maintained through its cutaneous photosynthesis and oral ingestion. By some estimates, one billion people worldwide have vitamin D deficiency or insufficiency. A number of factors influence the photosynthesis and bioavailability of vitamin D and contribute to risk of impaired vitamin D status. These factors include variation in sun exposure due to latitude, season, time of day, atmospheric components, clothing, sunscreen use and skin pigmentation, as well as age, obesity and the incidence of several chronic illnesses. This review will focus on factors that influence vitamin D status and contribute to the prevalence of low vitamin D levels. Key words: vitamin D; vitamin D synthesis; ultraviolet irradiation; sun exposure; vitamin D metabolism; vitamin D bioavailability; vitamin D deficiency.
(Accepted June 27, 2010)
Acta Derm Venereol 2011; 91: 115–124.
Martin A. Weinstock, MD, PhD, Dermatoepidemiology Unit, VA Medical Center-111D, 830 Chalkstone Ave, Providence, RI 02908, USA. E-mail: maw@brown.edu
Vitamin D is a steroid hormone with pleiotropic actions on most tissues and cells in the body (1, 2). The active form of the vitamin, 1,25-dihydroxyvitamin D [1,25(OH)2D], plays an essential role in calcium and phosphorus homeostasis, bone mineralization and skeletal growth. More recently, vitamin D status has been linked to cancer, cardiovascular disease, autoimmune disease and infection. While the definition of optimal vitamin D status remains controversial, levels of the predominant circulating form of this important vitamin, 25-hydroxyvitamin D [25(OH)D], lower than the commonly recommended optimum of 75 nmol/l (30 ng/ml) (3), remain a major public health issue. According to current estimates, one billion people worldwide have suboptimal circulating 25(OH)D levels (2). A number of biological and environmental factors combine to influence vitamin D status in humans. This review will focus on our current understanding of these various factors and briefly discuss their contribution to vitamin D status in specific populations.
Synthesis and Metabolism of Vitamin D
Work from the 1920s and 1930s led to the discovery that vitamin D could be synthesized endogenously in mammalian skin exposed to ultraviolet (UV) radiation (4). The mechanism and sequence of chemical reactions involved in this process were elucidated in 1980 (5). Photoproduction of vitamin D begins with synthesis of the sterol provitamin D3 molecule 7-dehydrocholesterol. In most vertebrate animals, including humans, this is produced in large quantities in the skin and incorporated into plasma membrane lipid bilayers of cells in the dermis and epidermis. When the skin is exposed to sunlight, 7-dehydrocholesterol absorbs UVB radiation in the wavelength range 290–315 nm. The absorbed energy causes chemical bonds within the 7-dehydrocholesterol molecule to break and rearrange, resulting in the formation of previtamin D3. In the skin, previtamin D3 undergoes rapid, thermally-induced transformation to vitamin D3.
Once formed, previtamin D3 and vitamin D3 continue to absorb UV radiation in a wide range of wavelengths. This sometimes results in breakdown of these molecules into biologically inert photoproducts (6). For this reason, during prolonged exposure to UV radiation, a steady state is reached in which only 10–15% of cutaneous 7-dehydrocholesterol is converted to previtamin D3 (7). It has been suggested that this process of photoregulation ensures that toxic levels of vitamin D3 are not synthesized under conditions of excessive sun exposure (8).
Cutaneously synthesized vitamin D3 is released from the plasma membrane and enters the systemic circulation bound to vitamin D-binding protein (DBP) (8). Serum concentrations of vitamin D3 peak 24 to 48 h following exposure to UV radiation (9). Thereafter, vitamin D3 levels decline exponentially with a serum half-life ranging from 36 to 78 h (9, 10). As a lipid-soluble molecule, vitamin D3 can be taken up by adipocytes and stored in subcutaneous or omental fat deposits for later use (11). The distribution of vitamin D3 into adipose tissue prolongs its total-body half-life to approximately two months (12).
Circulating vitamin D3 is metabolized in the liver, by the enzyme vitamin D-25-hydroxylase, to 25(OH)D3. This is the major circulating form of vitamin D and the molecule typically measured by clinicians wishing to assess vitamin D status. The rate and extent of the elevation of serum 25(OH)D3 levels following UV irradiation or vitamin D3 ingestion are dependent on the regulated activity of vitamin D-25-hydroxylase and are thus variable (see Metabolism of Vitamin D section below). The serum half-life of 25(OH)D3 is approximately 15 days (12). 25(OH)D3 is not biologically active except at very high, non-physiological levels (13). Activation requires its conversion to 1,25(OH)2D3 in the kidney and other organs by the enzyme 25(OH)D-1α-hydroxylase. Production of 1,25(OH)2D3 is tightly regulated by a number of factors, the most important of which are serum phosphorus and parathyroid hormone (PTH) levels (8). Catabolism of 1,25(OH)2D3 is also tightly regulated (see Metabolism of Vitamin D section below), but turnover is typically rapid with estimates of its serum half-life ranging from 3.5 to 21 h (14, 15).
Factors Influencing Cutaneous Vitamin D Synthesis
Exposure to ultraviolet radiation
Any process that alters the amount of UVB radiation entering the skin may significantly affect vitamin D3 production. UVB radiation is the portion of the electromagnetic spectrum between 280 nm and 320 nm. The UV “action spectrum” shows the relative effectiveness of UV radiation of different wavelengths at producing a biological response. For cutaneous vitamin D3 synthesis, the action spectrum falls within the UVB range. Optimum wavelengths for vitamin D3 production are between 295 nm and 300 nm, with production peaking at 297 nm (6).
The amount of vitamin D3-effective UVB radiation that reaches the earth’s surface is influenced by a number of factors. As UV radiation passes through the earth’s atmosphere, ozone (O3) in the stratosphere or at ground level absorbs all wavelengths below 280 nm. (It also absorbs longer wavelengths, but with decreasing efficiency.) A 12.5% decrease in atmospheric O3, as commonly occurs in specific regions over time, results in an approximate 15% increase in the monthly amount of vitamin D3-effective UVB radiation that reaches the earth’s surface (16).
UVB can be absorbed, scattered, or reflected by many additional substances as it travels through the earth’s atmosphere, including oxygen and nitrogen, aerosols, water vapour, particulate pollutants and cloud matter (16). For example, black carbon particulates generated by the combustion of fossil fuels and biomass reduce surface radiation by up to 5% in a typical urban environment (17), while extensive biomass burning, such as that which occurs in the rainforests of Brazil, results in local reductions of UVB radiation of up to 81% (18). Even in the absence of significant pollution, columns of water vapour within thick cloud cover can reduce surface UVB radiation to 1% of clear-sky levels, causing vitamin D3 synthesis to cease, even at the equator (19).
Another key factor influencing UVB radiation is the solar zenith angle (SZA). The SZA is the angle between the local vertical (zenith) and a line from the observer to the sun. Smaller SZAs (which occur when the sun is high in the sky) result in more intense UV radiation. This is due to two distinct processes. First, when UV radiation strikes a surface at an angle, its incident energy is spread over a larger surface area. Second, larger SZAs force the UV radiation to travel through a greater portion of the earth’s atmosphere to reach a given location (20). Time of day, time of year and latitude combine to establish the SZA for a specific point and time. In general, incident UVB radiation levels reach a maximum at mid-day in the summer (20). Below a latitude of approximately 35° North, UVB radiation is sufficient for vitamin D3 synthesis all year round. At higher latitudes, vitamin D3 is not produced during the winter months (8). For example, in Rome, Italy (latitude 41.9° North), cutaneous vitamin D3 synthesis is not possible from November through February. Ten degrees further north in Berlin, Germany (latitude 52.5° North) or Amsterdam, Netherlands (latitude 52.4° North), vitamin D3 synthesis ceases between October and April (21).
The large number of variables influencing UVB exposure makes it difficult to estimate vitamin D synthesis at a given place and time. To deal with this complexity, researchers have developed models that simulate UV radiation under various conditions and translate exposures into amount of vitamin D produced. For example, Engelsen & Kylling (22) developed the FastRT UV simulation tool, which computes surface irradiances (radiant energy per unit area) in the spectral range 290–400 nm as a function of SZA, ozone content, cloud and aerosol optical thickness, surface reflectance and cloud constellations. The calculated product of these irradiances is then integrated with the action spectrum for the conversion of 7-dehydrocholesterol to previtamin D3 in human skin to compute vitamin D3-effective UV doses (23). Effective UV doses are equated to oral vitamin D3 doses using the estimate that exposure of 25% of the body surface area to 25% of the personal minimal erythema dose (MED) increases vitamin D levels by the same amount as an oral dose of 1000 IU (1 MED is defined as the lowest dose of UV radiation for an individual that causes faint pink coloration of the skin within four distinct borders 24 h post-exposure). This estimate of oral dose equivalence is extrapolated from data showing that whole-body exposure to 1 MED of UV radiation produces a rise in serum vitamin D levels equivalent to that produced by an oral dose of approximately 16,000 IU (20).
There are a number of limitations with the computer modeling technique described above. With regard to the equivalence formula used by Engelsen et al., the data it is based on come from two different studies with a combined total of only 15 subjects (9, 24). In each of the studies, several key subject characteristics are not defined. Factors such as age and body mass index have significant effects on the synthesis and bioavailability of vitamin D (see below). Without this information, particularly in light of the large variation in responsiveness of an individual’s vitamin D status to either oral ingestion or UV exposure (25), the relevance of equivalence calculations to human populations is not clear. Furthermore, Lo et al. (24) found that altering the vehicle in which oral vitamin D was administered from an ethanolic solution to a capsule resulted in a 1.5- to 2-fold reduction in peak serum vitamin D concentrations following ingestion [calculated from data in Figs 1 and 2 of ref. (24)]. Re-extrapolation of the Engelsen et al. formula assuming oral ingestion of vitamin D not in ethanol, but in capsule from (a far more common form of supplement), yields an oral equivalence of one whole-body MED of 24,000 IU instead of 16,000 IU. It should also be noted that the formula is based on the assumption that the relationship between exposed surface area and change in serum vitamin D3 levels is linear. Published data do not adequately justify this assumption. In addition, Adams et al. (9) looked at changes in serum vitamin D3 concentrations after whole-body exposure to 1 MED of UV radiation. While studies have shown that repeated suberythemal (< 1 MED) doses of UV radiation can increase levels of circulating 25(OH)D3 (26, 27), a linear relationship between suberythemal UV radiation dose and change in serum vitamin D3 levels has not been established. Finally, model calculations presented in publications are typically generated using a specific set of conditions and are based on UV irradiation of a horizontal surface. Actual UV exposures for a given individual will vary dramatically minute-to-minute as atmospheric conditions and body position change. In light of these factors and the very large variation in dose-response, it is difficult at this time to quantify the effect of specific measures on vitamin D status without direct measurement of the patient’s vitamin D levels.
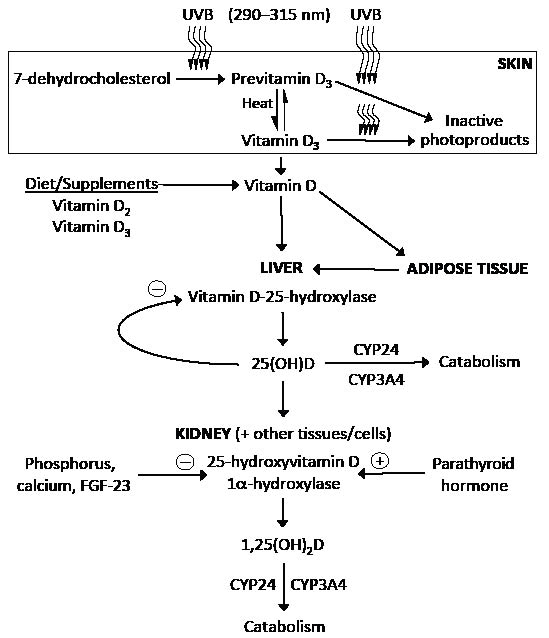
Fig. 1. Synthesis and metabolism of vitamin D. 7-dehydrocholesterol (provitamin D3) in the skin absorbs ultraviolet B (UVB) radiation with wavelengths of 290–315 nm and is converted to previtamin D3. Previtamin D3 undergoes thermal isomerization to vitamin D3. Continued exposure to UVB radiation can result in the breakdown of previtamin D3 and vitamin D3 to inactive photoproducts. Dietary vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol) are absorbed in the gastrointestinal tract, incorporated into chylomicrons, and transported via the lymphatic system into systemic circulation. Vitamin D (vitamin D2 and vitamin D3) from the diet and skin enters the circulation bound to the vitamin D-binding protein. As a fat-soluble molecule, it can be taken up by adipose tissue and stored for later use. Circulating vitamin D is metabolized in the liver to 25-hydroxyvitamin D [25(OH)D] by the enzyme vitamin D-25-hydroxylase. Vitamin D-25-hydroxylase activity is inhibited by 25(OH)D (negative feedback). 25(OH)D re-enters the circulation and is metabolized in the kidney and other tissues to the active metabolite 1,25-dihydroxyvitamin D [1,25(OH)2D] by 25(OH)D-1α-hydroxylase. Renal production of 1,25(OH)2D is inhibited by elevated serum levels of phosphorus, calcium and fibroblast growth factor 23 (FGF-23). Parathyroid hormone enhances renal production of 1,25(OH)2D. Catabolism of 25(OH)D and 1,25(OH)2D into biologically-inactive molecules is primarily mediated by the cytochrome P-450 enzymes CYP24 and CYP3A4.
Cutaneous factors
Prior to interacting with 7-dehydrocholesterol, levels of UVB energy reaching the sites of vitamin D synthesis are further attenuated by factors such as clothing and sunscreen, and influenced by the skin’s melanin content. The capacity of apparel textiles to interfere with UV radiation depends on a number of specific fabric qualities. Lightweight, non-synthetic fibers such as cotton and linen are less effective at blocking UV radiation than wool, silk, nylon and polyester (28). Bleaching of cotton cloth results in transmission of approximately 24% of incident UV radiation, whereas the same unbleached cotton fabric transmits only 14.4% (29). As one might expect, dense woven fabrics transmit significantly less UV radiation then loose-knit ones (30). Each of these textile qualities has the potential to influence the amount of vitamin D3-effective UVB radiation reaching the skin. For example, one study looking at the effect of different white and black fabrics on UV exposure found that black wool reduced UVB irradiance by 98.6%, while white cotton reduced it by only 47.7%. However, both of these fabrics completely suppressed vitamin D3 synthesis in vitro following 40 min of simulated sunlight or in volunteers subjected to whole body irradiation with up to 6 MEDs of UV radiation (31).
Topical sunscreen agents interfere with UVB–7-dehydrocholesterol interactions by absorbing, reflecting or scattering incident UV radiation. In vitro, the application of 5% (w/v) para-aminobenzoic acid (PABA) sunscreen to skin samples prevented photoconversion of 7-dydrocholesterol to previtamin D3. In the same study, a single, whole-body application of PABA sun protection factor (SPF) 8 blocked the elevation of serum vitamin D3 levels following exposure to 1 MED (32). A sunscreen’s SPF is effectively a measure of its capacity to protect against UV-induced erythema (33). By definition, SPF is the ratio of the MED with the tested sunscreen to the MED without it. This definition is based on the application of 2 mg/cm2 of sunscreen product (33). Typical amounts of sunscreen used are considerably lower then this. One study found that typical sunscreen application was only 0.5 mg/cm2, with only 43% of individuals reapplying sunscreen at appropriate intervals (34). Nevertheless, a study of 20 long-term sunscreen users in the U.S. states of Illinois and Pennsylvania found that their mean serum 25(OH)D3 level was 40 nmol/l, versus 91 nmol/l in 20 age- and sun exposure-matched control subjects (35). In a larger, randomized controlled trial conducted in Australia, however, researchers observed little difference between placebo and sunscreen groups in terms of either mean 25(OH)D3 levels or change in 25(OH)D3 levels over the summer months (36).
Melanin is a large opaque polymer that is produced constitutively and in response to UV radiation by melanocytes in the skin. Melanin efficiently absorbs electromagnetic radiation across the entire UV and visible light range and thus competes with 7-dehydrocholesterol for UVB photons (33). Compared to individuals with lightly-pigmented skin, those with high concentrations of melanin (darkly-pigmented skin e.g., African Americans) require longer UV exposure times to generate an equivalent amount of vitamin D3 (37). At the population level, African American women were found to be more than 20-fold more likely than Caucasians to have serum 25(OH)D3 levels < 25 nmol/l (12.2% versus 0.5% of the respective study populations) (38).
Levels of 7-dehydrocholesterol strongly influence cutaneous vitamin D3 synthesis. Post-burn scar tissue was found to contain only 42.5% of the 7-dehydrocholesterol typically found in normal skin, and in the absence of supplementation burn patients often develop progressive vitamin D deficiency (39). There is also an age-related decline in skin 7-dehydrocholesterol content. The average concentration of 7-dehydrocholesterol in the epidermis of 77–88-year-olds is 65% lower then that in 21–29-year-olds [calculated from data in Table I of ref. (40)]. Perhaps as a consequence, a second study reported vitamin D3 synthesis following whole-body exposure to simulated sunlight to be approximately 78% lower in 62–80-year-olds than in 20–30-year-olds (41).
Finally, skin temperature plays an important role in cutaneous vitamin D3 synthesis. Conversion of photosynthesized previtamin D3 to vitamin D3 is a temperature-dependent isomerization process (5). The rate of the isomerization reaction correlates directly with skin temperature. A study using the skin of the lizard Iguana iguana, whose rate constant for the isomerization of previtamin D3 to vitamin D3 is similar to that of human skin, showed that isomerization occurs 9-fold more quickly at 25°C than at 5°C [50% of previtamin D3 converted to vitamin D3 (T1/2) in 8 and 72 h, respectively] (42). At 37°C, the T1/2 for the conversion of previtamin D to vitamin D in human skin decreases to 2.5 h (43). Skin temperature at any given location on the surface of the body is dependent on a number of variables, including blood perfusion, thermal conductivity, metabolic activity, insulation by clothing, ambient temperature, air-flow rate, air pressure and humidity (44). Under most normal conditions, human skin temperature is lower than core body temperature and varies between approximately 29°C and 35°C (45). The rate of cutaneous vitamin D synthesis will, in turn, vary as skin temperature fluctuates.
Bioavailability of Vitamin D After Oral Ingestion or Cutaneous Synthesis
Gastrointestinal absorption of vitamin D
Following cutaneous synthesis or oral consumption, vitamin D bioavailability is dependent on intestinal absorption, fat storage and metabolism. Dietary vitamin D consists of vitamin D2 (ergocalciferol) derived from non-vertebrate species (invertebrates, fungi and plants) and vitamin D3 (cholecalciferol) derived from vertebrates. Vitamins D2 and D3 differ only slightly in their chemical structure and both have been used to effectively treat suboptimal vitamin D status, vitamin D deficiency rickets and osteomalacia (46–49). There is, however, some controversy as to the biological equivalence of these two forms of vitamin D, with some studies suggesting they are differently metabolised (49, 50), and others reporting differential effects on serum 25(OH)D levels (51–53). Vitamins D2 and D3 are both relatively non-polar molecules and must therefore be solubilized by incorporation into bile-salt micellar solutions in order to be absorbed to the aqueous phase. Absorption occurs primarily in the proximal small intestine and is influenced by gastric, pancreatic and biliary secretions, micelle formation, diffusion through the unstirred-water layer, brush-border-membrane uptake, and transport out of the intestinal cell (24). Any process resulting in malabsorption of intestinal fat may impair the absorption of vitamin D. In one study, absorption of tritium-labeled (3H)-vitamin D in normal subjects ranged from 62.4% to 91.3% of the initial oral dose (10). In patients with celiac disease, biliary obstruction absorption and chronic pancreatitis, absorption fell to 50%, < 28% and < 18% of the oral dose, respectively. In each case, impaired vitamin D absorption correlated with the degree of steatorrhea. Other conditions in which vitamin D absorption is impaired include liver failure (see below), cystic fibrosis, Crohn’s disease, and gastric bypass. Individuals taking bile acid-binding medications (such as colestyramine and colestipol for hypercholesterolemia) will also have impaired vitamin D absorption (2, 24).
Obesity
Vitamin D obtained from the diet or cutaneous synthesis is readily taken up by adipose tissue, which, it has been suggested, stores vitamin D for subsequent release and metabolism at times when production is reduced, such as during the winter months (11). Levels of adipose tissue, however, appear to inversely correlate with vitamin D status. Several studies have shown that obese individuals tend to have lower serum concentrations of vitamin D3 and 25(OH)D3 than those with normal weights (54–58). For example, in one study, whole-body irradiation produced an increase in serum vitamin D3 levels in obese individuals [body mass index (BMI) 30 kg/m2] that was 57% lower than that in age-matched, normal weight controls (BMI 25). In the same study, peak serum vitamin D2 concentrations after intake of an oral dose of 50,000 IU of vitamin D2 inversely correlated with BMI (54). Thus, obesity is associated with decreased bioavailability of dietary and cutaneously synthesized vitamin D. This is likely secondary to the sequestration of vitamin D into a larger pool of adipose tissue (2).
Metabolism of vitamin D
Specific enzymes involved in the metabolism of vitamin D, 25(OH)D and 1,25(OH)2D play important roles in regulating the bioavailability of these molecules. The active form of vitamin D, 1,25(OH)2D, is produced by the enzyme 25(OH)D-1α-hydroxylase. This enzyme is tightly regulated by serum phosphorus and PTH levels such that serum levels of 1,25(OH)2D are stable across wide ranges of ingested or synthesized quantities of vitamin D. For example, in vitamin D deficiency, as assessed by serum 25(OH)D concentration, levels of 1,25(OH)2D may be normal or even elevated due to secondary hyperparathyroidism (2). Inactivating mutations in the gene encoding 25(OH)D-1α-hydroxylase cause pseudovitamin D deficiency rickets (vitamin D-dependent rickets type 1), in which little or no renal 1,25(OH)2D synthesis occurs (59).
Conversion of vitamin D to 25(OH)D is mediated by the enzyme vitamin D-25-hydroxylase. The activity of this enzyme is directly inhibited by 25(OH)D. This negative feedback mechanism helps maintain serum concentrations of 25(OH)D within a restricted physiological window – 75–220 nmol/l – in the face of significant variation in vitamin D ingestion and synthesis (13). Another effect of this negative feedback loop is that the rate of increase in serum 25(OH)D for a given oral dose of vitamin D is inversely related to the starting level of 25(OH)D. At low starting 25(OH)D levels (< 50 nmol/l), the average rate of increase is estimated at 1.2 nmol/l for every 40 IU of oral vitamin D consumed daily. In severely deficient states (< 10 nmol/l), this rate can reach 3.45 nmol/l for every 40 IU of vitamin D (60). At higher starting levels [> 70 nmol/l 25(OH)D], the rate of increase drops to 0.7 nmol/l for every 40 IU of oral vitamin D consumed daily (61–63).
Catabolism of 25(OH)D and 1,25(OH)2D is primarily mediated by two cytochrome P-450 enzymes. CYP24 [also known as 25(OH)D-24-hydroxylase] initiates the breakdown of 25(OH)D and 1,25(OH)2D in the kidney and, to a lesser extent, other tissues, while CYP3A4 mediates their catabolism in the liver and small intestine (64). The combined activity of these two enzymes is an important factor in determining the circulating concentrations of 25(OH)D and 1,25(OH)2D (65). Of clinical relevance is the fact that long-term use of certain medications, including phenobarbital, phenytoin, carbamazepine, rifampicin and antiretrovirals (HAART), causes up-regulation of CYP3A4. This leads to decreased levels of 25(OH)D and 1,25(OH)2D, often resulting in clinically significant osteomalacia (2, 64, 66–70).
Other Diseases That Affect Vitamin D Status
Kidney disease
The enzyme 25(OH)D-1α-hydroxylase is responsible for production of the majority of circulating 1,25(OH)2D within the proximal tubules of the kidney. As a result, renal pathology can significantly affect vitamin D status. Chronic kidney disease, defined as structural or functional abnormalities of the kidney that last for at least three months, affects approximately 20 million adults within the U.S. (71). In chronic kidney disease, creatinine clearance is positively correlated with serum 1,25(OH)2D levels (72, 73). With disease progression, declining glomerular filtration rates are associated with a corresponding decrease in 1,25(OH)2D levels, such that levels of 1,25(OH)2D are usually undetectable in end-stage renal disease (74). Regulation of renal 25(OH)D-1α-hydroxylase activity occurs through PTH-induced enzyme synthesis and direct stimulation of enzymatic activity by PTH and hypophosphatemia (75, 76). Impaired kidney function secondary to chronic kidney disease results in phosphate retention. The resulting hyperphosphatemia is a potent inhibitor of renal 25(OH)D-1α-hydroxylasee activity. Loss of functioning kidney mass also results in decreased levels of this enzyme and consequent deficiency in circulating 1,25(OH)2D (77). In addition to this, low levels of 25(OH)D can occur in nephrotic-range proteinuria due to direct loss of vitamin DBP-bound 25(OH)D in the urine (74).
Liver disease
The liver plays an important role in the maintenance of vitamin D status, and hepatobiliary disease is often associated with low levels of 25(OH)D and impaired bone metabolism (78). In cholestatic liver disease, there is a decrease in the intestinal availability of bile salts. This results in malabsorption of fat-soluble vitamins such as vitamin D. In one study, six children who had suffered from chronic cholestasis since infancy (mean age 12.1 years) displayed a 98.7% lower mean rise in serum vitamin D2 levels following a single oral dose of 1000 IU per kg than controls (79). In severe parenchymal liver disease, there is both vitamin D malabsorption and reduced capacity for its 25-hydroxylation, leading to 25(OH)D deficiency. A study of 100 patients with noncholestatic chronic liver disease found that 86.3% of cirrhotic patients were vitamin D-deficient [serum 25(OH)D level < 50 nmol/l] compared to only 49.0% of noncirrhotic controls (80). The liver is also the site of synthesis for vitamin DBP, the primary carrier protein for vitamin D, 25(OH)D and 1,25(OH)2D in the circulation. DBP binding prolongs the half-life of vitamin D and its metabolites and facilitates their uptake by target tissues (76). Decreased levels of serum DBP have been observed in patients with fulminant hepatic failure, in whom low DBP levels are associated with increased mortality (81). Low DBP concentrations are also seen in chronic liver disease due to the liver’s diminished synthetic capacity (82).
Granulomatous disorders and malignancies
Hypercalcemia secondary to elevated serum 1,25(OH)2D levels frequently occurs in granulomatous disorders such as sarcoidosis, tuberculosis, fungal granulomas and berylliosis (83). The increase in serum 1,25(OH)2D stems from the extra-renal conversion of 25(OH)D to 1,25(OH)2D in activated macrophages (84, 85). The reported incidences of hypercalcemia in granulomatous disorders vary widely. This is due in part to the fact that the hypercalcemia is often transient and dependent on disease activity. For example, in sarcoidosis, the incidence of hypercalcemia varies from 2% to 63%, with most large studies reporting incidences of approximately 10%. Although commonly reported, the incidences of hypercalcemia and elevated serum 1,25(OH)2D levels in other granulomatous disorders have not been as thoroughly investigated (86).
Tumour-induced osteomalacia (also known as oncogenic hypophosphatemic osteomalacia) is a para- neoplastic syndrome characterized by hypophosphatemia secondary to renal phosphate wasting. A biochemical hallmark of this syndrome is low 1,25(OH)2D levels (despite pronounced hypophosphatemia), leading to severe rickets or osteomalacia (87). The syndrome is typically associated with tumours of mesenchymal origin, but has been seen with epithelial tumours such as breast and prostate carcinomas (88). It is caused by tumour secretion of fibroblast growth factor 23 (FGF-23) (89). Although the precise mechanism by which FGF-23 induces osteomalacia is not known, it has been shown to reduce the synthesis of sodium-phosphate co-transporters, thereby contributing to the observed renal phosphate wasting. It has also been shown to suppress renal 25(OH)D-1α-hydroxylase expression and to upregulate CYP24 expression, leading to decreased production and increased degradation of 1,25(OH)2D (90). Removal of FGF-23-producing tumors results in rapid (within 30-min) reduction in circulating FGF-23 levels and, depending on the severity of the bone disease, a slower normalization of serum phosphate and 1,25(OH)2D levels (87).
Prevalence of LOW Vitamin D Status
The results of a number of studies suggest the optimal serum 25(OH)D concentration for both skeletal and non-skeletal health outcomes is approximately 75 nmol/l (3) (although this value may vary according patient characteristics). Vitamin D deficiency is commonly defined as a serum 25(OH)D concentration less than 20–25 nmol/l and insufficiency defined as a serum 25(OH)D concentration between 25 and 75 nmol/l. Vitamin D deficiency and insufficiency defined in this way are common and continue to be public health issues (2, 13). In the National Health and Nutrition Examination Survey (NHANES) 2001–2004, analysis of 25(OH)D concentrations in over 20,000 U.S. individuals found that approximately 30% met the above criteria for vitamin D deficiency or insufficiency (91). During childhood, severe vitamin D deficiency causes rickets, a disease in which growing bone fails to mineralize, resulting in skeletal abnormalities, short stature, delayed development and failure to thrive. The prevalence of nutritional rickets in the U.S. is estimated to be between 5 and 9 cases per 1 million children (59). Recently, 9% of the U.S. pediatric population, a figure representing 7.6 million children and adolescents, were found to have serum 25(OH)D concentrations less then 37.5 nmol/l, potentially placing them at increased risk of developing rickets (92).
In adults, low levels of vitamin D are associated with osteomalacia and the precipitation or exacerbation of osteopenia and osteoporosis, as well as an increased risk of fractures (93). Older adults are often considered at increased risk of vitamin D deficiency due to limited sun exposure, decreased capacity for cutaneous vitamin D synthesis (40, 41) and reduced intake of dietary vitamin D (1). According to the NHANES 2001–2004 data, approximately 30% of U.S. adults aged > 50 years had serum 25(OH)D concentrations lower then 50 nmol/l (91).
Individuals living at higher latitudes and/or with darker skin are likely to have lower levels of vitamin D because of reduced vitamin D3-effective UV radiation. A study in the U.S. State of Maine (latitude approximately 44° North) found that 48% of preadolescent girls tested had serum 25(OH)D concentrations < 75 nmol/l by the end of the winter and 17% following the summer months (94). In nearby Boston, Massachusetts (latitude 42° North), 36% of African American adolescents and 22% of Hispanic adolescents were found to have serum 25(OH)D levels lower then 37.5 nmol/l (95). In a study of pregnant women from The Hague, Netherlands (latitude 52° North), meanwhile, minority Muslim women had substantially lower mean serum 25(OH)D levels than Western women, perhaps due to a combination of differences in skin tone and UV exposure (96). As we have seen, UV irradiance is not the only determinant of vitamin D status. Individuals living at lower latitudes in relatively sunny environments are also at risk of vitamin D insufficinecy. Studies in Hawaii (97), South Florida (98), Southern Arizona (99), Brazil (100), rural India (101) and Queensland, Australia (102) found that significant proportions of the study populations had low vitamin D levels despite abundant sun exposure.
Conclusions
A number of factors are known to influcene vitamin D levels and it is likely that there exist others that are yet to be identified. Further research is needed to fully elucidate the role each of these factors plays in regulating levels of vitamin D and its metabolites in populations at risk of vitamin D deficiency and insufficiency. There continues to be some debate as to the optimal strategy for improving vitamin D status. Some experts have recommended that increased sun/UVB exposure is an effective and inexpensive approach to maintaining adequate vitamin D levels (2, 103), although that point of view has been criticized (104). Ultraviolet radiation is a known human carcinogen linked to the development of both melanoma and non-melanoma skin cancers (1). The ultraviolet wavelengths responsible for cutaneous vitamin D synthesis, skin erythema (sunburn) and DNA damage all fall in the UVB range. Thus UVB-induced vitamin D production cannot be dissociated from the processes of photo-damage and photo-carcinogenesis (1, 9, 105). For this reason, continued protection from sun exposure, and maintenance of optimal vitamin D status through increased oral intake of vitamin D, have been advocated (106). The optimal public health strategy with respect to vitamin D status remains controversial and may involve increasing oral consumption and/or fortifying foods with vitamin D.
The authors declare no conflict of interest.
References
