Hong Liang Tey
National Skin Centre, 1, Mandalay Road, Singapore, Singapore
Hong Liang Tey
National Skin Centre, 1, Mandalay Road, Singapore, Singapore
Hypopigmentation disorders in children can be due to a wide variety of congenital and acquired diseases. A clinical approach to hypopigmentation disorders based on the typical age of onset and the extent of lesions is proposed. The disorders are categorized into onset in early and later childhood, and in each category they are subdivided into localized and generalized pigmentary disorders. Clinical findings, comprising the sites of involvement, degree of pigment loss, and associated morphological findings, are used to distinguish the disorders further. This classification provides a systematic approach to a clinical condition in which the causes are heterogeneous and histological examination of the skin is rarely diagnostic. Key words: approach; childhood; pediatric; hypopigmentation; hypopigmented.
(Accepted October 26, 2009.)
Acta Derm Venereol 2010; 90: 6–11.
Hong Liang Tey, National Skin Centre, 1, Mandalay Road, Singapore 308205, Singapore. E-mail: teyhongliang111@yahoo.com
The colour of the skin is mainly due to melanin and blood but can be altered in non-physiological conditions such as carotenemia, drug intake, jaundice and chronic renal failure. Hypopigmentation refers to any form of decreased pigmentation, whereas hypomelanosis refers specifically to a decrease in melanin content. Depigmentation, in contrast to hypopigmentation, describes the almost total loss of pigmentation, resulting in a whitish appearance that comes from the underlying dermis.
Hypopigmentation disorders can generally be categorized based on their aetiologies, age of onset, and extent of involvement. In early childhood, many of these disorders have a genetic origin and present with generalized pigmentary dilution. In later childhood, many of these diseases are acquired and cause localized hypopigmentation. Other clinical features can aid in the further differentiation of these disorders, such as the sites of involvement, degree of pigment loss, and associated morphological signs. In the clinical approach to be presented here, the disorders are categorized primarily according to the typical age of onset into early and later childhood.
Onset in early childhood
In this article, disorders that present in early childhood describe those disorders that occur up to the first 2 years of life. Most of these disorders present at birth or during infancy and have a genetic basis. Clinically, these disorders can be divided into those characterized by generalized hypopigmentation and those causing localized disease.
Generalized hypopigmentary disorders
Disorders with generalized diffuse pigmentary dilution are usually due to mutations of genes responsible for the production of melanin or the processing of melanosomes, and hypopigmentation of the skin and hair is present. During embryogenesis, progenitor melanoblasts migrate between mesodermal and ectodermal layers to reach their final destinations in the epidermis and hair follicular bulbs, as well as the inner ear cochlea, choroids, ciliary body, and iris (1). Therefore, the eyes, in addition to the skin and hair, can exhibit pigmentary dilution. In a group of generalized hypopigmentary disorders, hypopigmentation involves the eyes in addition to the skin and hair, and this group has been typified as oculocutaneous albinism (Fig. 1).
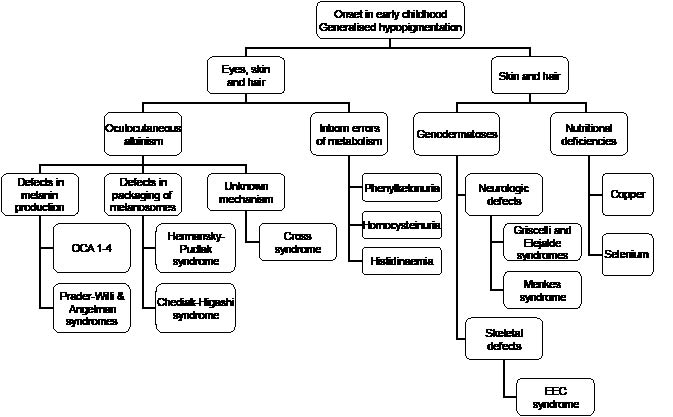
Fig. 1. Generalized hypopigmentation with early onset.
Oculocutaneous albinism (OCA) is the most common type of pigmentary dilutional disorder, of which type 2 comprises the majority of the cases. In OCA type 1A, in which there is complete absence of tyrosinase activity, there is permanent and complete absence of pigment from birth. In OCA types 1B, 2, 3, and 4, however, pigment production may increase over time. In these latter types of OCA and including Hermansky–Pudlak and Chediak–Higashi syndromes, the pigmentary dilution may be subtle, and comparison with family members will be helpful. This is especially so in fair-skinned races.
Common to all types of OCA, there is reduced visual acuity and ocular nystagmus (2) and these distinguish OCA from other forms of congenital hypopigmentation. The ocular abnormalities are due to misrouting of the optic nerve and this may result from deficiency of tyrosinase. Tyrosine hydroxylase, in particular, is important in the proper routing of retinal projections at the optic chiasm during development (3). Infants who are unable to fixate or have nystagmus should be sent for a thorough ocular examination as early intervention is crucial for visual development. In addition, hypopigmented babies should undergo a systemic evaluation to exclude other rarer disorders. For example, the presence of mental retardation suggests Angelman and Prader–Willi syndromes, an obese baby may have Prader–Willi syndrome, bleeding diatheses may be a sign of Hermansky–Pudlak syndrome, immune deficiency points to Chediak–Higashi syndrome, and structural ocular and neurological abnormalities suggest Cross syndrome.
Impairment of melanin synthesis occurs in certain diseases of inborn errors of metabolism, such as phenyl- ketonuria, homocystinuria and histidinaemia, which are due to the absence or defect in phenylalanine hydroxylase, cystathionine synthetase, and histidase, respectively. These three disorders are transmitted in an autosomal recessive manner and are associated with mental retardation and other systemic dysfunction.
Some genetic disorders result in pigmentary dilution of the skin and hair, but spare the eyes. The patients should be screened for systemic abnormalities and if neurological defects are present, the differential diagnoses will include Griscelli, Elejalde and Menkes syndromes. Griscelli and Elejalde syndromes are rare, autosomal recessive disorders with abnormalities in the transport of lysosome-related organelles, which include melanosomes, platelet-dense bodies and lymphocyte lytic granules (4). Besides cutaneous pigmentatory dilution, the consequence of disrupted melanosome transfer is silver discoloration of the hair, which can be seen to contain multiple clumps of melanin under light microscopy. In fact, Griscelli, Elejalde and Chediak–Higashi syndromes have been termed “silvery hair syndromes” (5). Apart from pigmentary dilution and neurological abnormalities, Griscelli syndrome is also characterized by immunological impairment. There are three types of the disease known currently, and Elejalde syndrome has the same clinical features as Griscelli syndrome type 1. Elejalde syndrome, also known as neuroectodermal melanolysosomal disease, is characterized by silvery hair, mild hypopigmentation, and severe central nervous system dysfunction (6). Its molecular basis is currently not known.
Menkes syndrome is an X-linked recessive multi-systemic disorder due to inappropriate intracellular copper storage. It is typified by hair shaft abnormalities, of which pili torti is the most common. Pigmentary abnormalities consist of lightly pigmented hair and generalized or localized hypopigmentation. Progressive central nervous system deterioration occurs and results in death by 3 years of age (7).
Pigmentary dilution and skeletal defects occur in Ectrodactyly-Ectodermal dysplasia-Cleft lip/palate (EEC) syndrome. The “lobster-claw” deformities of the hands and feet and cleft lip and palate enable the syndrome to be recognized easily. The cutaneous features consist of diffuse hypopigmentation and dryness of the skin and hair (8).
Loss of hair and skin pigmentation due to selenium deficiency has been described in children receiving long-term total parenteral nutrition, and repigmentation occurred after selenium supplementation (9). Hypopigmentation of the skin and hair has also been described in severely malnourished infants, and this was attributed to copper deficiency because tyrosinase is a copper-dependent enzyme. However, as multiple nutritional deficiencies tend to co-exist, the pathogenesis was difficult to confirm (10).
In all of the disorders outlined in Fig. 1, the epidermis generally contains normal numbers of melanocytes and histology of the skin is not helpful in differentiating these conditions. The pathophysiological defect of hypopigmentation lies either in melanin biosynthesis or melanosome formation and trafficking. Disorder of melanin biosynthesis can be due to tyrosinase defects (occurring in OCA types 1 and 3 and copper deficiency) or melanosomal dysfunction (occurring in OCA types 2 to 4). Abnormalities in the formation, transport, and transfer of melanosomes occur in Hermansky–Pudlak, Chediak–Higashi, and Griscelli syndromes.
Localized hypopigmentary disorders
Localized hypopigmentation presenting in early childhood can be due to genetic or acquired causes. These disorders can clinically be categorized based on whether the lesions are depigmented or hypopigmented (Fig. 2).

Fig. 2. Localized hypopigmentation with early onset.
Piebaldism and vitiligo are both characterized by an absence of epidermal melanocytes and the clinical presentation of depigmentation. Unlike vitiligo, lesions in piebaldism are present at birth and almost always remain stable throughout life. There are usually normally pigmented to hyperpigmented skin as islands within the depigmented patches and at the lesional borders in piebaldism. The lesions are classically distributed over the mid-forehead, anterior trunk and mid-extremities, and they are hardly ever found near the midline on the back – this is due to the embryonic pattern of dorso-ventral migration of melanocytes. The c-KIT and SLUG genes have been found to be mutated in piebaldism. Dysfunction of the protein product, a tyrosine kinase transmembrane receptor on melanocytes, leads to aborted migration and survival of melanocytes in the skin (11).
In patients presenting with signs of piebaldism, it is important to check for features of Waardenburg syndrome (WS), which include heterochromia irides and a broad nasal root. In WS type 1, dystopia canthorum is present in addition to these features (12). In WS type 2, unlike the other types of WS, sensorineural deafness is common. WS type 3 consists of limb defects in addition to features of type 1 disease and WS type 4 consists of Hirschsprung disease in addition to features of type 1 disease. It is therefore important to screen for hearing and musculoskeletal defects and chronic constipation in an infant with dysmorphic features of WS. In WS, there is a failure of melanocytes to migrate and survive in the epidermis, hair follicles, irides and inner ear. Certain genes are involved in neural crest development and mutations of these genes in WS type 4 lead to defects of both melanocyte development in the various organs and neuronal development in the distal colon (leading to Hirschsprung disease or congenital aganglionosis). In WS type 2, the microphthalmia-associated transcription factor (MITF) gene is mutated and mutation of the same gene can also lead to Tietz syndrome. Tietz syndrome is clinically distinguishable from WS type 2 in that the former is characterized by a more severe phenotype, presenting with generalized, instead of patchy hypopigmentation and complete, instead of variable hearing loss (13).
In an infant who presents with a single hypopigmented macule or patch, the main differential diagnoses are nevus depigmentosus, an ash leaf macule of tuberous sclerosis, and nevus anemicus. Nevus anemicus is a localized vascular anomaly in which the vessels are hypersensitive to catecholamines and it can be distinguished from the hypomelanotic disorders in a few ways. Using diascopy, nevus anemicus can be made to blend into the surrounding blanched skin. Nevus anemicus is not accentuated by Wood’s lamp, in contrast to lesions which contain less melanin. The reflex vasodilatory response is also absent upon application of pressure and heat; scratching a line across nevus anemicus will not induce erythema in the lesion and the contrast can be seen in the surrounding normal skin.
The most common causes of a single hypomelanotic patch are nevus depigmentosus and an ash-leaf macule of tuberous sclerosis. The diagnosis of tuberous sclerosis becomes more likely if there are multiple hypomelanotic macules (only about 10% of patients with tuberous sclerosis present with a single hypomelanotic lesion) (14) or if the lesion is lance ovate-shape like ash leaves (rounded at one end and pointed at the other). Ash-leaf macules are usually the first manifestation of tuberous sclerosis and the other cutaneous features, such as facial angiofibromas, only start appearing after the age of 5 years (15). The diagnosis of tuberous sclerosis may therefore only be apparent when the child is older. Histologically, the primary finding in both nevus depigmentosus and ash-leaf spot is a decrease in epidermal melanin content. Ultrastructural differences have been described, consisting of a decrease in the transfer of melanosomes in nevus depigmentosus and a decreased number of melanosomes in tuberous sclerosis (16). The differentiation between nevus depigmentosus and tuberous sclerosis remains clinical.
Hypomelanosis of Ito is a descriptive term for streaks of hypopigmentation that presents in infancy. It is not a specific diagnosis and it may occur as a consequence of several different chromosomal abnormalities that perturb various genes relevant to skin pigmentation. Although the characteristic configurations are whorled and linear, the lesions can be patchy as well. On the other hand, there is a variant of nevus depigmentosus that consists of segmental or linear hypopigmentation and this overlap with hypomelanosis of Ito clinically. Some clinicians have use the term “naevoid linear hypopigmentation” to encompass both conditions (17). Upon the diagnosis of hypomelanosis of Ito, it is essential to exclude associated systemic abnormalities, which occurs in about one-third of children (18), affecting the central nervous and musculoskeletal systems and the heart particularly.
The lesions in hypomelanosis of Ito tend to follow the lines of Blaschko, which are believed to be pathways of migration and proliferation of epidermal cells during embryogenesis. The bands of abnormally pigmented skin represent clones of cells carrying a mutation in a gene expressed in the skin. Apart from somatic mutations, mosaicism following the lines of Blaschko is also seen in chromosomal mosaicism and functional mosaicism (random X-chromosome inactivation through lyonization). Clinically, these disorders present with linear pigmentary changes as one of their features. The linear or segmental variant of nevus depigmentosus, hypomelanosis of Ito, and linear and whorled hyperpigmentation are probably a phenotypic spectrum of such genetic mosaicism and have recently been classified under the term “pigmentary mosaicism”.
The important genodermatoses presenting with pigmentary dilution and hypopigmented lesions in the infancy period are listed in Table I. The disorders have been classified according to their pathogenetic defects in melanogenesis.
Table I. Underlying defects in genodermatoses with pigmentary dilution or hypopigmented lesions (from ref. 19)
| Defect | Disease | Gene | Locus |
| Proliferation and migration of melanocytes | Piebaldism | c-kit | 4q12 |
| Waardenburg syndrome (WS) | WS1 and WS3: PAX3 | 2q35 | |
| WS2A: MITF | 3p14.1–p12.3 | ||
| WS4: SOX10, EDN3, EDNRB | 22q13, 20q13.2–13.3, 13q22 | ||
| Tietz syndrome | MITF | 3p14.1–p12.3 | |
| Production of melanin | Oculocutaneous albinism (OLA) | OCA1: TYR | 11q14–q21 |
| OCA2: OCA2 | 15q11.2–q12 | ||
| OCA3: TYRP1 | 9p23 | ||
| OCA4: MATP | 5p13.3 | ||
| Prader-Willi and Angelman syndrome | OCA2 (gene causing hypopigmentation) | 15q21 | |
| Menkes syndrome | ATP7A | Xq13.2–13.3 | |
| Packaging of melanosomes | Chediak–Higashi syndrome | LYST | 1q42.1–q42.2 |
| Hermansky–Pudlak syndrome | Type 1: HPS1 | 10q23.1 | |
| Tuberous sclerosis | TSC1, TSC2 | 9q33–q34, 16p13.3 | |
| Transfer of melanosomes | Griscelli syndrome | Type 1: MYO5A | 15q21.1 |
| Type 2: RAB27A | 15q21.1 | ||
| Type 3: MLPH (or MYO5A F-exon del) | 2q37 | ||
| Incontinentia pigmenti | NEMO | Xq28 |
Onset in later childhood
Disorders which typically presents after the first one to two years of life are included here. Most of these are acquired in origin. Similar to those presenting in early childhood, these diseases can be clinically divided into those characterized by generalized hypopigmentation and those causing localized disease.
Generalized hypopigmentary disorders
Generalized hypopigmentation presenting in an older child is uncommon. The causes include vitiligo universalis (presenting with depigmentation), inborn errors of metabolism (homocysteinuria and histidinaemia), malnutrition (in particular copper and selenium deficiency), and a delay in the diagnosis of an infancy-onset pigmentary dilutional disorder. The causes and approach are presented in Fig. 3.
Fig. 3. Generalized hypopigmentation with late onset.

Homocysteinuria and histidinaemia (also known as histidinuria) usually present during early childhood. Central nervous system disorders are features of both diseases and musculoskeletal and cardiovascular abnormalities are also present in homocysteinuria.
Localized hypopigmentary disorders
Hypopigmentary disorders presenting in later childhood are usually localized. The common causes are presented in Fig. 4.

Fig. 4. Localized hypopigmentation with late onset.
Vitiligo is characterized clinically by depigmented macules and patches that correspond histologically to a decrease or absence of melanocytes in the epidermis and, less often, the hair follicles. Its most common age of onset is 10–30 years of age, with a mean age of 20 years. In general, vitiligo can be categorized based on the distribution into localized, generalized, and universal forms. Localized forms can be segmental, focal, or mucosal and generalized forms can be acrofacial or the vulgaris type. Established generalized and segmental forms are usually easily recognized as depigmented patches in their typical distribution and configuration, respectively. However, focal and mucosal types of vitiligo can be difficult to recognize, especially for early lesions that are not yet depigmented. The following, if present, can help support the diagnosis of vitiligo in ambiguous cases: leucotrichia, Koebner phenomenon, halo naevi, and associated autoimmune diseases, such as thyroid abnormalities and alopecia areata.
A common cause of post-inflammatory hypopigmentation in older children is pityriasis alba and this is typically limited to the face. Post-inflammatory hypopigmentation is also commonly seen in association with atopic dermatitis as well as with other forms of eczema. The decrease in pigmentation can be the result of a disruption in the transfer of melanin to keratinocytes secondary to the inflammatory process or the application of potent topical corticosteroids. In addition, post-inflammatory hypopigmentation in children can also be due to pityriasis lichenoides chronica and lichen striatus. When an older child presents with widespread 3–6 mm macular hypopigmentation, pityriasis lichenoides chronica should be considered. In lichen striatus, the hypopigmentation is typically linear following the lines of Blaschko and multiple small, flat-topped papules may be seen. Post-infectious hypopigmentation can result from superficial cutaneous infections as well as childhood viral exanthems and varicella. Post-traumatic hypopigmentation is also not uncommon in children due to the frequent superficial injuries they sustain.
If the hypopigmented lesions are associated with an atrophic epidermal surface, lichen sclerosus, morphea and the hypopigmented variant of mycosis fungoides are to be considered. Lichen sclerosus is a pruritic chronic inflammatory dermatosis that results in hypopigmented to depigmented porcelain white plaques, which are associated with epidermal atrophy and dermal induration. Up to 15% of cases occur in children, and the lesions in the majority of these cases occur at the vulva with absence of extragenital manifestations. Possible pathogenetic mechanisms in the development of this leukoderma include decreased melanin production, blocked transfer of melanosomes to keratinocytes, and loss of melanocytes (20). Lichen sclerosus and morphea have been considered to be closely related by many authors, but this remains controversial.
Hypopigmentation is not uncommonly seen in lesions of localized scleroderma (morphea) and systemic sclerosis. The lesions may mimic vitiligo and the clues to the diagnosis of scleroderma include the presence of perifollicular hyperpigmentation (forming a “salt and pepper” dyschromatosis) and induration of the dermis. The epidermal melanocytes in the interfollicular regions disappear, but those in the near vicinity of hair follicles are retained. This is in contrast to vitiligo in which melanocytes in both regions are affected. In re-pigmenting vitiligo, re-pigmentation typically starts in the perifollicular region, and differentiating it from scleroderma can sometimes be difficult.
The hypopigmented variant of mycosis fungoides (MF) is a rare condition in childhood or adolescence (21), but this variant seems to be more frequent in children compared to other types of MF (22). This condition should be considered if there are scattered irregular hypopigmented patches on non-sun exposed areas of the body and one should search for typical patch, plaque and tumour lesions of MF, which may be present concurrently. Histologically, epidermotropism of atypical lymphocytes may be seen together with a decrease or absence of epidermal melanin and pigmentary incontinence.
Investigations
Investigations are dependent on the diagnoses suspected. In congenital hypopigmentary disorders, karyotyping and molecular genetic analysis of the skin and blood may be considered, especially when the child has associated developmental delay or structural abnormalities. In conditions which exhibit mosaicism, skin biopsy plays a more important role in the diagnosis and blood tests are of limited use. However, the advanced molecular techniques required for diagnosis of these congenital disorders are not widely available. Biopsy of hypopigmented lesions for histology in general is rarely diagnostic, but this is important in acquired disorders where inflammatory disorders or mycosis fungoides is suspected.
Management
In general, treatment for many hypopigmented disorders is limited, especially those which are congenital in origin. Hypopigmented diseases with an associated inflammatory component may be treated with topical corticosteroids and calcineurin inhibitors when lesions are limited and with phototherapy when lesions are widespread and the child is older. Autologous grafting of cultured and non-cultured melanocytes has been used successfully to treat stable vitiligo and piebaldism (23).
The importance of sun protection, including the use of a broad-spectrum sunscreen on hypopigmented lesions, should be emphasized to patients and parents. Hypopigmented lesions are more susceptible to sun damage and the lesions will be more obvious with the differential tanning response compared with the surrounding normal skin. In addition, Koebner phenomenon may arise in lesions of vitiligo.
Cosmetic cover-ups for camouflage may be the only option in some hypopigmented disorders. Topical stains and tanning products may be used on the lesions to decrease the colour disparity with the normal skin, and most of these products contain dihydroxyacetone as the active ingredient.
The psychosocial aspect of the patient is an important integral part of management. This is particularly so in schoolchildren, when the hypopigmentary disorder can be the source of significant embarrassment and psychological trauma to the developing child. Co-operation and regular communication between the physician, parents and school is essential, and enrolling the help of counsellors and child psychiatrists should be instituted early when appropriate.
Conclusion
Hypopigmentation disorders in children can be due to a wide variety of congenital and acquired diseases. A clinical approach to hypopigmentation disorders based on the typical age of onset of disease and the extent of the lesions is presented here. Further distinction of the disorders is dependent on the clinical findings, comprising the sites of involvement, degree of pigment loss and associated morphological findings. A systematic approach will be useful for this clinical condition, the causes of which causes are heterogeneous, and histological examination of the skin is often non-diagnostic.
The author declares no conflict of interest.
References
