Kyung Ho Lee1, Jung Eun Kim1, Baik Kee Cho1, Yu Chan Kim2 and Chul Jong Park1
Department of Dermatology, 1College of Medicine, The Catholic University of Korea, and 2Ajou University School of Medicine, Suwon, Korea
Kyung Ho Lee1, Jung Eun Kim1, Baik Kee Cho1, Yu Chan Kim2 and Chul Jong Park1
Department of Dermatology, 1College of Medicine, The Catholic University of Korea, and 2Ajou University School of Medicine, Suwon, Korea
Patients with the autosomal-dominant form of multiple familial trichoepithelioma develop numerous tumours on the face, neck and upper trunk, beginning in childhood. Malignant transformation of such lesions is quite rare; only one case of “malignant trichoepithelioma” has been reported previously, inferring pilomatrix carcinoma on a histological observation. We report here the case of a patient who developed a malignant neoplasm in a long-standing trichoepithelioma lesion on her buttock. Histopathology revealed a transformation zone between the trichoepithelioma and a malignant tumour mass. This case also showed several features of a malignant neoplasm of trichoblastic origin. Key words: trichoblastic carcinoma; malignant transformation; trichoepithelioma; trichoblastoma.
(Accepted April 24, 2007.)
Acta Derm Venereol 2008; 88: 43–46.
Chul Jong Park, Department of Dermatology, College of Medicine, The Catholic University of Korea, 2, Sosa-Dong, Wonmi-Gu, Pucheon-Si, Kyunggi-Do, 420-717, Korea. E-mail: cjpark777@yahoo.co.kr
In contrast to the various benign follicular differentiated neoplasms, there are only a few malignant hair follicle tumours. Pilomatrix carcinoma and trichoblastic carcinoma are the only malignant hair follicle tumours that have been well established as entities up to now (1). The hypothesis that trichoblastic carcinoma develops from trichoblastoma has been debated (2–3). We present here a case of trichoblastic carcinoma, supporting earlier reports of development of trichoblastic carcinoma via malignant transformation of trichoblastoma.
Case report
A 35-year-old Korean woman presented with numerous, dome-shaped, pin-head to pea-sized, translucent and whitish papules and nodules enlarging slowly on the whole body, which had developed since childhood. A livid, 7×6.5×3 cm, ulcerative mass on the buttock had enlarged suddenly 2 months earlier (Fig. 1A, B). Her father and 2 elderly sisters had similar, but less severe, lesions. The patient was otherwise in good health. A malignant tumour on the buttock was suspected and staging work-up was performed, including chest and abdominal computerized tomography (CT), pelvic magnetic resonance imaging (MRI), bone scan and tumour markers. There was no evidence of systemic metastasis, and a wide excision was performed on the buttock mass. The histopathology findings of the punch biopsy specimen from her arm showed basophilic basaloid tumour islands and horn cysts, arranged in a lace-like pattern with solid aggregates, consistent with trichoepithelioma. An excisional biopsy specimen from the buttock mass showed a large, asymmetric, poorly circumscribed, infiltrative tumour with adjacent benign trichoepithelioma in the overall dermis. A transformation zone was observed clearly between the trichoepithelioma and malignant tumour mass (Fig. 2A). The malignant tumour consisted of nests and cords of neoplastic cells, and the peripheral tumour cells showed frequent cytological atypia, mitotic figures and central transitions toward clear or occasionally squamoid appearing cells, which resembled cells of the follicular outer root sheath (Fig. 2B–C). There was also an area of extensive necrosis and focal area in continuity with the epidermis (Fig. 2D–E). Immunohistochemical staining demonstrated focal expression of CD34 in the stromal cells surrounding the malignant tumour nests and within the papillary mesenchymal body in the benign portion. These findings led to a diagnosis of trichoblastic carcinoma. The mass was excised and there was no evidence of distant metastasis or recurrence during a 6-month follow-up.
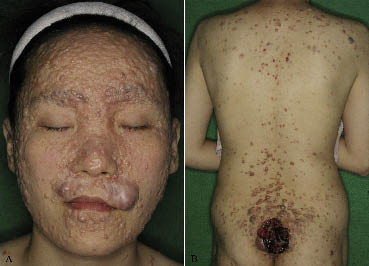
Fig. 1. Asymptomatic, numerous, dome-shape papules and nodules (A) on the face and (B) on the whole body as well as an asymmetric livid large mass with ulceration on the buttock.

Fig. 2. (A) Poorly circumscribed neoplasm involving the dermis and subcutis. A transitional zone is clearly observed between the benign and the malignant neoplastic cell nests (H&E staining, original magnification ×100). (B) Atypical clear cells resembling keratinocytes of the outer root sheath are shown (H&E staining, original magnification ×400). (C) Basaloid tumour islands with organization simulating hair-germ surrounded by outer sheath-type cells and perifollicular stroma show focal infiltrative growth (H&E staining, original magnification ×100). (D) Malignant trichoblasts are in continuity with surface epithelium (H&E staining, original magnification ×40). (E) Necrosis en masse in the neoplastic cell nests, which show a trabeculae-like growth pattern (H&E staining, original magnification ×100).
Discussion
A trichoblastic carcinoma is a rare malignant neoplasm with differentiation toward follicular germinative cells with a similarity to a trichoblastoma. In total, 12 cases of malignant follicular tumours have now been reported in the literature (1–8) (Table I). These cases were divided into low- and high-grade neoplasm according to the level of clinical malignancy. The former usually shows locally invasive growth and frequent recurrence. The latter shows more destructive features and the capability of systemic metastasis. Two groups of trichoblastic carcinomas have the following features in common: older person, large, solitary, asymmetric, poorly circumscribed, dermal or subcutaneous mass, often in continuity with the surface or infundibular epidermis, at the base of a long-standing trichoblastoma, poorly-differentiated carcinomas with high mitotic activity, infiltrative growth and necrosis en masse (1–8). The distinguishing features of a high-grade neoplasm are a sudden enlargement with inflammation, large size (> 3 cm), location on the trunk and extremities and capability of systemic metastatic spread (1, 3, 7). Low-grade trichoblastic carcinomas appear to be slowly enlarging, smaller than high-grade, plaques or nodules occur on the face, and do not recur if excised completely (2, 4–6). Among the cases of low-grade trichoblastic carcinoma shown in Table I, the case reported by Altman et al. (4) was referred to as a “plaque variant of trichoblastic fibromas” and the case reported by Cowen et al. (5) was originally named an “unusual aggressive trichoblastoma”. However, these cases are similar to subsequent reports of low-grade trichoblastic carcinomas and are best classified as low-grade trichoblastic carcinomas (2, 8).
Table I. Clinical characteristics of trichoblastic carcinomas
|
Ref. |
Age/sex |
Location |
Duration (years) |
Diagnosis |
Size (cm) |
Clinical features |
Prognosis |
|
Low-grade |
|||||||
|
Altman et al., 1995 (4) |
62/F |
Chin |
38 |
Trichoblastic fibroma |
2.5×2.5 |
Slowly enlarging yellowish firm plaque |
No evidence of recurrence during 2 years |
|
Altman et al., 1995 (4) |
61/F |
Cheek |
NA |
Trichoblastic fibroma |
1.3×0.7 |
Slowly growing, yellowish to erythematous, indurated plaque |
No evidence of recurrence during 2 years |
|
Altman et al., 1995 (4) |
66/F |
Nose |
NA |
Trichoblastic fibroma |
2.5×2.0 |
Slowly growing, yellowish to erythematous, indurated plaque |
No evidence of recurrence during 2 years |
|
Cowen et al., 2000 (5) |
47/M |
Chin |
5 |
Trichoblastoma |
2.0×2.6 |
Slowly growing pearly firm plaque |
No evidence of recurrence during 2 years |
|
Rofagha et al., 2001 (2) |
53/F |
Lip |
Several |
Trichoblastoma |
2×1 |
Slowly enlarging nodule |
NA |
|
Rofagha et al., 2001 (2) |
74/M |
Lip |
2~3 |
Trichoblastoma |
1.6×0.7 |
Slowly enlarging nodule |
NA |
|
Ayhan et al., 2006 (6) |
73/F |
Nasal alar |
1 |
Trichoblastoma |
1 |
Slowly growing, blue-gray pigmented nodule |
NA |
|
High-grade |
|||||||
|
Sau et al., 1992 (7) |
73/F |
Abdomen |
40 |
Trichoblastoma |
4 |
Rapid growth and inflammation in the past few weeks |
Death due to systemic metastases 4 months after excision |
|
Regauer et al., 2000 (3) |
55/M |
Arm |
40 |
Trichoblastoma |
3.5 |
Sudden enlargement and livid discoloration |
Death due to systemic metastases 21 months after excision |
|
Shulz et al., 2005 (1) |
84/M |
Forearm |
4~5 |
Trichoblastoma |
3 |
Enlargement and livid discoloration over past 6 weeks |
No recurrence or metastases within 2 years |
|
Shulz et al., 2005 (1) |
87/M |
Thigh |
3 |
Trichoepithelioma |
4 |
Enlargement and livid discoloration over several weeks |
No recurrence or metastases within 26 months |
|
Our case, 2006 |
35/F |
Buttock |
2~3 |
Trichoepithelioma |
7×6.5 |
Sudden enlargement and livid discoloration |
No recurrence or metastases within 6 months |
NA, not available.
There has been some controversy as to whether a malignant transformation of trichoblastoma actually occurs. Regauer et al. (3) suggested that the tumour may have arisen de novo in a patient predisposed to the formation of follicular tumours. On the other hand, it may have resulted through a malignant degeneration of a pre-existing benign neoplasm. Schulz et al. (1) recently reported a case of trichoblastic carcinoma at the base of a trichoepithelioma in a patient with Brooke-Spiegler syndrome. They also documented a clearly visible transitional zone between this malignant follicular tumour and the adjacent trichoepithelioma, as in our case.
The main differential diagnosis of this malignant follicular neoplasm is a basal cell carcinoma with matrical differentiation, squamous cell carcinoma, pilomatrix carcinoma and trichilemmal carcinoma (1, 9, 10). Ackerman & Gottlieb (11) proposed that a basal cell carcinoma be termed a trichoblastic carcinoma. However, this concept has yet to be fully accepted.
The malignant epithelial tumour described here resembled neither a conventional basal cell carcinoma nor a squamous cell carcinoma. Instead, the tumour cells were made up of large cells with abundant cytoplasm rather than small basaloid cells, which is the typical feature of a basal cell carcinoma. There was no peripheral palisading of neoplastic cells and no cleft between the tumour lobule and the surrounding stroma (1). A squamous cell carcinoma must also be taken into consideration when the mainly abundant and somewhat amphophilic cytoplasm of these neoplastic cells are observed. However, this tumour did not show the key features of a squamous cell carcinoma, such as intercellular bridges, squamous eddies, or areas with a glassy cytoplasmic appearance (1). Likewise, a pilomatrix carcinoma could be ruled out due to the lack of the typical shadow cells, which is mandatory for a diagnosis of pilomatrix carcinoma (9). In addition, a trichilemmal carcinoma can be ruled out because of the frequent occurrence only on sun-protected skin (10), which is unusual in Koreans.
In summary, this case shared the features of a trichoblastic carcinoma and there was an apparent transitional zone between primary trichoepithelioma and malignant neoplasm. Therefore, this case was named as a “malignant transformation of multiple familial trichoepithelioma”. High-grade trichoblastic carcinomas that develop from a pre-existing lesion, as in our patient, require careful diagnostic attention, staging work-up and close follow-up.
References
1. Schulz T, Proske S, Hartschuh W, Kurzen H, Paul E, Wunsch PH. High-grade trichoblastic carcinoma arising in trichoblastoma: a rare adnexal neoplasm often showing metastatic spread. Am J Dermatopathol 2005; 27: 9–16.
2 Rofagha R, Usmani AS, Vadmal M, Hessel AB, Pellegrini AE. Trichoblastic carcinoma: a report of two cases of a deeply infiltrative trichoblastic neoplasm. Dermatol Surg 2001; 27: 663–666.
3 Regauer S, Beham-Schmid C, Okcu M, Hartner E, Mannweiler S. Trichoblastic carcinoma (”malignant trichoblastoma“) with lymphatic and hematogenous metastases. Mod Pathol 2000; 13: 673–678.
4. Altman DA, Mikhail GR, Johnson TM, Lowe L. Trichoblastic fibroma. A series of 10 cases with report of a new plaque variant. Arch Dermatol 1995; 131: 198–201.
5. Cowen EW, Helm KF, Billingsley EM. An unusually aggressive trichoblastoma. J Am Acad Dermatol 2000; 42: 374–377.
6. Ayhan M, Gorgu M, Aytug Z, Karatas Silistreli O, Ozkan S, Ermete M. Trichoblastic carcinoma of the alar region: a case report. Dermatol Surg 2006; 32: 976–979.
7. Sau P, Lupton GP, Graham JH. Trichogerminoma. Report of 14 cases. J Cutan Pathol 1992; 19: 357–365.
8. Helm KF, Cowen EW, Billingsley EM, Ackerman AB. Trichoblastoma or trichoblastic carcinoma? J Am Acad Dermatol 2001; 44: 547.
9. Hunt SJ, Abell E. Malignant hair matrix tumor (”malignant trichoepithelioma“) arising in the setting of multiple hereditary trichoepithelioma. Am J Dermatopathol 1991; 13: 275–281.
10. Laochumroonvorapong P, Kokta V, Quan MB. Trichilemmal carcinoma in an African American. Dermatol Surg 2002; 28: 284–286.
11. Ackerman AB, Gottlieb GJ. Fibroepithelial tumor of pinkus is trichoblastic (basal-cell) carcinoma. Am J Dermatopathol 2005; 27: 155–159.
