Jochen Schmitt1, Knut Schäkel1, Natalie Schmitt2 and Michael Meurer1
1Department of Dermatology, University Hospital Carl Gustav Carus, and 2Research Association Public Health Saxony, Medical Faculty Carl Gustav Carus, Technical University, Dresden, Germany
Systemic immunosuppressive agents are recommended for patients with atopic eczema in whom disease activity cannot be controlled adequately with topical treatments. Guidelines do not give clear advice which agents to prefer. We systematically reviewed clinical trials on systemic treatment for severe atopic eczema to provide evidence-based treatment recommendations. Standardized literature search, independent standardized assessment of eligibility and data abstraction was performed by 2 reviewers. Twenty-seven studies totalling 979 patients were included. Eleven studies consistently showed effectiveness of cyclosporine. Cyclosporine is recommended as first option for patients with atopic eczema refractory to conventional treatment. Evidence from randomized controlled trials also exists for interferon-γ and azathioprine. Although frequently used in clinical practice, systemic glucocorticosteroids have not been assessed adequately in studies. Mycophenolate mofetile showed effectiveness in 2 small uncontrolled studies. Intravenous immunoglobulins and infliximab are not recommended based on published data. Key words: atopic dermatitis; evidence-based medicine; immunosuppressive therapy; immunomodulator; systemic treatment.
(Accepted October 10, 2006.)
Acta Derm Venereol 2007; 87: 100–111.
Jochen Schmitt, Department of Dermatology, University Hospital Carl Gustav Carus, Technical University Dresden, Fetscherstr. 74, DE-01307 Dresden, Germany. E-mail: jochen.schmitt@uniklinikum-dresden.de
With a prevalence of up to 20% in children and 1–10% in adults living in industrialized countries, atopic eczema (AE) is among the most common dermatological conditions (1–4). AE imposes a high economic burden, both in terms of total cost and out-of-pocket expenses (5, 6). Although most cases of AE are mild in terms of objective clinical activity, this condition adversely affects most aspects of everyday life in the majority of patients (7–9). Most patients can be treated effectively with emollients and topical anti-inflammatory agents such as topical corticosteroids and the topical calcineurin inhibitors (1, 10).
There is a broad consensus that topical treatments should be used as first-line therapy. Systemic treatment modalities are limited to the subgroup of patients in whom the activity of skin lesions and concurrent symptoms cannot be controlled sufficiently with conventional topical treatments and phototherapy (10–12). For those patients published treatment guidelines recommend agents such as systemic glucocorticosteroids, cyclosporin A (CyA), methotrexate, azathioprine (AZT), interferon-g (IFN), intravenous immunoglobulin (IVIG) and mycophenolate mofetile (MMF) (10, 11).
Recommendations are based on small randomized controlled trials (RCT) or, more frequently, on uncontrolled studies, case reports and expert opinion. Different systemic treatment options have not yet been compared against each other in a RCT.
We performed a systematic review of prospective studies on systemic treatment options for patients with severe AE who could not be controlled adequately with conventional topical therapies. Our primary objective was to provide evidence-based recommendations on which systemic immunosuppressive or immunomodulatory agent to use as first and second choice treatment for these patients.
MATERIALS AND METHODS
We systematically reviewed all prospective clinical studies on the effectiveness of systemic immunosuppressive/immunomodulatory drugs in patients with severe AE. To minimize selection bias due to different baseline severity we limited our review to studies evaluating the subset of patients with severe AE, who do not adequately respond to topical treatments.
Literature search
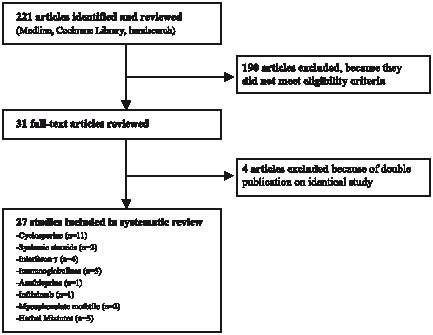
A standardized electronic literature search was performed using MEDLINE (until August 2005) and the keywords “(atopic AND (eczema OR dermatitis)) OR neurodermatitis”, for study type “(study OR trial OR comparison) AND (treatment OR drug OR therapy)”. Specific treatment options were identified by searching for the generic names of immunosuppressive / immunomodulatory drugs discussed in current treatment guidelines (10, 11). We limited the literature search to papers on humans, papers with abstracts, and excluded reviews. A total of 213 articles matched these criteria. Eight additional papers were identified in the Cochrane Skin Group specialized register and the Cochrane central register of controlled trials and by hand-searching the reference lists of review articles on AE (Fig. 1).
Study selection
Each of these 221 articles was reviewed for eligibility by 2 independent reviewers (JS, NS) using a standardized eligibility form. Disagreements were resolved by discussion. Exclusion criteria comprised no original data reported, studies not carried out in humans, no diagnosis of AE, only subgroup of patients with AE included (e.g. extrinsic AE), no systemic treatment, patients not classified as inadequately controlled by conventional therapies, no clinical end-point, no prospective study, case reports/case series on less than 5 patients, and no full-text article (e.g. letter). A total of 31 articles met the eligibility criteria, 4 of which were secondary publications on studies that have been published previously (13–16). Thus, 27 studies were included in this systematic review (17–43) (Fig. 1).
Fig. 1. Identification of relevant studies for inclusion in the systematic review.
Data extraction and quality assessment
Twenty-seven articles were abstracted using standardized data extraction and quality assessment forms. Relevant data of each study was independently extracted by 2 reviewers (JS, NS). Disagreements were resolved by consensus. Recorded data included information on study population (geographical region, number of patients enrolled, age range, inclusion criteria regarding the severity of AE), year of publication, study design (study type, dosage and duration of active treatment), concurrent treatment, clinical outcome measure (investigator-rated measurement including intensity and extent of skin lesions, if assessed), study result, safety, and study quality assessment.
Effectiveness was expressed as change in mean objective clinical severity (defined as investigator-rated measurement including intensity and extent of skin lesions) from baseline to end of active treatment. If not mentioned in the paper, the mean relative change in clinical severity was calculated using absolute scores at baseline and during treatment. In some articles the mean absolute severity scores were not reported in the text, but could be derived from a presented figure or graph. If means were not reported, the distribution of relative individual responses was abstracted. To be able to compare RCT and non-controlled studies we considered exclusively the active treatment groups of placebo-controlled studies. In cross-over RCT we considered only the study period prior to cross-over. This was done to avoid information bias due to carry-over effects. Methodological quality was assessed in terms of adequate case definition, use of validated outcome, follow-up rate of 80% or more, conduct of intention-to-treat analysis, adequateness of randomization concealment and blinding procedures (44). If no information was provided, the corresponding quality item was judged inadequate. Since quality assessment is subjective and because it is not easy to distinguish between study quality and reporting quality, we did not exclude studies that did not meet certain quality criteria. Both data abstraction and quality assessment was based solely on the methods and results sections.
As surrogate variables for drug safety, serious adverse events and withdrawals due to adverse events were abstracted. To be comparable across studies, safety data is provided in events per month of immunosuppressive/immunomodulatory treatment. Primarily because of small case numbers and short follow-up periods, most RCT or uncontrolled effectiveness studies are inappropriate to assess adverse drug reactions (ADR) with long latency or rare events. Additional problems derive from varying and non-standardized reporting of ADRs in clinical studies. Therefore, the presented data on safety should be interpreted with caution.
RESULTS
Overall, 27 studies met all eligibility criteria, totalling 979 patients with severe AE, inadequately controllable with topical therapies (17–43) (Fig. 1). Tables I–III detail these studies. Among those, 11 studies on CyA, totalling 498 patients were identified. The corresponding data for other treatments were: systemic glucocorticosteroids (2 studies; n = 47), IFN (4 studies; n = 216), IVIG (3 studies; n = 25), MMF (2 studies; n = 20), AZT (1 study; n = 37), infliximab (1 study; n = 9), Chinese herbal therapy (CHT) (3 studies; n = 127) (Table I).
Table I. Characteristics of studies included in the systematic review
| Ref. Year | Study design | Country Number enrolled (Age range years) | Inclusion criteria regarding disease severity | Drug Duration of active treatment | Initial dose, comparator (if applicable) | Dose adjustments | Concurrent treatment | ||
| 37 1991 | d-b RCT | UK n = 33 (17–56) | Inadequately controlled by conventional therapies | CyA 8 weeks | 5 mg/kg BW vs. placebo | None | Topical steroids | ||
| 38 1994 | d-b RCT | Netherlands n = 46 (17–68) | Resistant to conventional therapies | CyA 6 weeks | 5 mg/kg BW vs. placebo | None | Antihistamines | ||
| 17 1996 | open RCT | Netherlands n = 78 (18–70) | Resistant to conventional therapy and/or significantly disabling AE | CyA 1 year | 3 mg/kg BW vs. 5 mg/kg BW | After 2 weeks stepwise adjustment to minimum effective dose | Topical steroids, antibiotics, antihistamines | ||
| 19 1996 | open u-c study | UK n = 27 (2–16) | Refractory to topical steroids | CyA 6 weeks | 5 mg/kg BW | None | Topical steroids, antihistamines | ||
| 18 1997 | open u-c study | UK n = 100 (≥12) | Disabling AE, inadequately controlled by topical steroids | CyA 48 weeks | 2.5 mg/kg BW | After 8 weeks stepwise adjustment to minimum effective dose | Topical steroids, antihistamines | ||
| 20 2000 | open RCT | UK n = 43 (2–16 ) | Refractory to topical steroids | CyA 1 year | 5 mg/kg BW; 12 weeks short courses vs. 1 year continuous therapy | After 4 weeks stepwise adjustment to minimum effective dose | Topical steroids | ||
| 31 2000 | d-b RCT | Germany n = 106 (≥18) | Refractory to conventional therapies and BSA 30% or more | CyA 8 weeks | 150 mg vs. 300 mg | After 2 weeks stepwise adjustment to minimum effective dose | Topical steroids, antihistamines | ||
| 21 2000 | open u-c study | Italy n = 10 (17–45) | Resistant to conventional therapies | CyA 6 weeks | 5 mg/kg BW | None | Not reported | ||
| 23 2001 | open u-c study | Germany n = 10 (1–15) | SCORAD > 50 and refractory to topical steroids | CyA 8 weeks | 2.5 mg/kg BW | After 2 weeks stepwise adjustment to minimum effective dose | Topical steroids | ||
| 22 2001 | open u-c study | Italy n = 15; 35.5 (median) | Resistant to conventional therapies | CyA 8 weeks | 5 mg/kg BW | Not reported | Not reported | ||
| 30 2004 | d-b RCT | Italy n = 30 (13–45) | Inadequately controlled by topical steroids | CyA 6 weeks | 3 mg/kg BW vs. topical tacrolimus 0.1% | None | Antihistamines | ||
| 35 1984 | d-b RCT | UK n = 27 (3–14) | Inadequately controlled by conventional therapies | Beclomethasone- diproprionate 4 weeks | 0.8 mg/day oral + 0.4 mg/day nasal | None | Topical steroids, antihistamines | ||
| 42 1995 | d-b RCT | Italy n = 20 (2–6 ) | Inadequately controlled by topical therapies | Flunisolide 2 weeks | 0.64 mg/day (age 2 years) 1.2 mg/day (age 3–6 years) | None | Antihistamines | ||
| 36 1993 | d-b RCT | USA n = 83 (2–65) | Inadequately controlled by conventional therapies | INF-g 12 weeks | 1.5 × 106 IU/m2/day vs. placebo | None | Systemic and topical steroids, antihistamines | ||
| 25 1993 | open u-c study | Germany n = 14 (22–33) | Inadequately controlled by topical steroids | INF-g 6 weeks | 5 × 2 × 106 IU in 1st week 3 × 2× 106 IU in week 2–4 2 × 2 × 106 IU in week 5–6 | None | None |
| Ref. Year | Study design | Country Number enrolled Age range (years) | Inclusion criteria regarding disease severity | Drug Duration of active treatment | Initial dose, comparator (if applicable) | Dose adjustments | Concurrent treatment | ||
| 24 1998 | open u-c study | Korea n = 68 (not stated) | Inadequately controlled by conventional therapies | INF-g 6 weeks | 5 × 106 IU/m2 in 1st week 3 × 106 IU/m2 in week 2–4 2 × 106 IU/m2 in week 5–6 | None | None | ||
| 39 2000 | d-b RCT | Korea n = 51 (≥15) | Inadequately controlled by conventional therapies, BSA > = 20% | INF-g 12 weeks | 1.5 × 106 IU/m2 3 × / week vs. 0.5 × 106 IU/m2 3 × weekly vs. placebo | None | None | ||
| 26 1998 | open u-c study | USA n = 9 (7–64) | Inadequately controlled by conventional therapies | IVIG 7 months | 2 g/kg BW / month | None | Systemic and topical steroids, antihistamines | ||
| 40 2002 | e-b RCT | France n = 10 (21–38 ) | SCORAD > 50 and inadequately controlled by conventional therapies | IVIG 1 cycle (evaluation at day 30) | 2 g/kg within 2 days (immediate or delayed treatment (at day 31)) | None | Topical steroids | ||
| 27 2002 | open u-c study | UK n = 6 (≥18) | Inadequately controlled by conventional therapies | IVIG 6 months | 2 g/kg BW / month | None | Systemic/topical steroids, azathiorine antihistamines, | ||
| 29 2000 | open u-c study | Germany n = 10 (29–47) | Inadequately controlled by conventional therapies | MMF 12 weeks | 1g/day in week 1 2g/day in week 2–12 | None | Topical steroids | ||
| 28 2001 | open u-c study | Germany n = 10 (19–66) | Inadequately controlled by conventional therapies | MMF 8 weeks | 2g/day in week 1–4 1 g/day in week 5–8 | None | Topical steroids in week 1–2; none in week 3–8 | ||
| 41 2002 | d-b RCT | UK n = 37 (17–73) | Inadequately controlled by topical steroids | Azathioprine 12 weeks | 2.5 mg/kg BW vs. placebo | None | Topical steroids | ||
| 43 2005 | open u-c study | Germany n = 9 (19–61) | Resistant to conventional therapies | Infliximab 10 weeks (primary end-point) | 5 mg/kg BW at weeks 0, 2, and 6 | None | Topical steroids | ||
| 33 1992 | d-b RCT | UK n = 40 (19–57) | Extensive (> 20% BSA) and refractory disease | CHT 8 weeks | Standardized formulation of 10 herbs (Zemaphyte) vs. placebo | None | Topical steroids | ||
| 34 1992 | d-b RCT | UK n = 47 (1–18) | Resistant to conventional therapies | CHT 8 weeks | Standardized formulation of 10 herbs (Zemaphyte) vs. placebo | None | None | ||
| 32 1999 | d-b RCT | Hong Kong n = 40 (7–50) | Inadequately controlled by topical treatment | CHT 8 weeks | Standardized formulation of 10 herbs (Zemaphyte) vs. placebo | None | Topical steroids |
AE: atopic eczema; BSA: body surface area; BW: body+weight; CHT: Chinese herbal therapy; c-o: cross-over; d-b: double-blind; u-c: uncontrolled; e-b: evaluator-blinded; CyA: cyklosporin A; INF-g: interferon-gamma; IVIG: intravenous immunoglobulim; IU: international units; MMF: mycophenolate mofetile; RTC: randomized controlled trial; SCORAD: Scoring Atopic Dermatitis Index (70).
Twenty-five studies were performed in Europe, 2 in the USA, 2 in Korea, and one in Hong Kong. Sample size varied considerably ranging from 9 to 106 patients. The majority of studies (n = 21; 78%) included less than 50 patients (Table I). Thirteen studies (48%) included only adults (age > 16), 5 studies (18%) exclusively children (age ≤ 16), 7 studies (26%) both children and adults, and 2 studies (7%) did not report the age range of patients included (Table I).
Fourteen studies (52%) were RCT, 7 of which were double-blind placebo-controlled cross-over RCT (32–35, 37, 41, 42), 3 were double-blind placebo-controlled parallel group RCT (36, 38, 39), 2 were open-label parallel group RCT comparing different dosing regimens of CyA (17, 20), one was a double-blind parallel group RCT comparing CyA and topical tacrolimus (30) and one was a double-blind parallel group RCT comparing different dosing regimens of CyA (31). One trial was a randomized evaluator-blinded uncontrolled study (40). The remaining 12 trials were open uncontrolled studies (18, 19, 21–29, 43).
Concomitant therapy with topical glucocorticosteroids was allowed by 18 study protocols (17–20, 23, 26–29, 31–33, 35–37, 40, 41, 43), 3 of which also permitted concomitant therapy with systemic glucocorticosteroids (26, 27, 36). Four studies did not allow any concomitant therapy except emollients (24, 25, 34, 39), 3 additionally allowed oral antihistamines (30, 38, 42).
An objective investigator-assessed disease severity score including intensity and extent of AE lesions was applied in 20 studies (74%), in 7 of which (35%) unnamed and non-validated scales were used. The remaining 13 studies applied a total of 5 different (original or modified) published severity scales (Table II). Extent and intensity of AE was assessed separately by means of non-validated scores in 5 studies. Only patient-assessed rating of extent or only investigator-assessed global disease severity was used in one study each. This wide variation in outcome methodology is a major source of heterogeneity.
Table II. Summary of results of studies included in the systematic review
| Ref. Year | Treatment | Outcome measure (clinical disease severity) | Results* | Safety Serious adverse events* Withdrawals due to adverse events* (n; % / month treatment) |
| 37 1991 | CyA | Non-validated score including intensity and extent (mean change) | 56% reduction in mean severity score | Abdominal pain (n = 1) 0; 0 |
| 38 1994 | CyA | Non-validated score including intensity and extent (mean change) | 55% reduction in mean severity score | Not reported 1; 2.9 |
| 17 1996 | CyA | Non-validated score including intensity and extent (mean change) | 46% vs. 29% reduction in mean severity score at week 2 in high-dose vs. low-dose group | Herpes simplex infection (n = 1); Acute cholecystitis (n = 1); both in low-dose group low-dose: 3; 0.9 high-dose: 3; 0.9 |
| 19 1996 | CyA | SASSAD (mean change) | 57% reduction in mean SASSAD | Not reported 1; 2.5 |
| 18 1997 | CyA | SASSAD (mean change) | 39% reduction in mean SASSAD | Viral infection (n = 1); basal cell carcinoma (n = 1) 14; 1.3 |
| 20 2000 | CyA | SASSAD (AUC of mean scores) | About 50% reduction in mean SASSAD in both groups | 17 events reported, but explicit information only on one case of folliculitis 2; 0.5 |
| 31 2000 | CyA | Non-validated score including intensity and extent (mean change) | 58% vs. 48% reduction in mean severity in high vs. low-dose group | None low-dose: 0; 0 high-dose: 3; 2.8 |
| 21 2000 | CyA | Costa’s Index (mean change) | 54% reduction in mean Costa’s Index | Not reported Not reported |
| 23 2001 | CyA | SCORAD (mean change) | 58% reduction in mean SCORAD | Not reported 0; 0 |
| 22 2001 | CyA | Extent on 4-point Likert scale (assessed by patient) (mean change) | About 90% reduction in mean extent score | Not reported 0; 0 |
| 30 2004 | CyA | SCORAD (mean change) | Similar effectiveness in both treatment groups. cyclosporin group: 88% reduction in mean SCORAD | None 0; 0 |
| 35 1984 | Beclomethasonediproprionate | Non-validated score including intensity and extent (mean change) | 22% decrease in mean severity score | Not reported 1; 0.9 |
| 42 1995 | Flunisolide | Non-validated score including intensity and extent (mean change) | 39% decrease in mean severity score | None 0; 0 |
| 36 1993 | INF-g | Non-validated intensity score and BSA separately (mean change), no composite severity score | About 30% reduction in mean intensity of lesions, no significant differences between verum and placebo | Not reported 1; 0.8 |
| 25 1993 | INF-g | Non-validated score including intensity, extent, and pruritus (relative individual response) | 58% improved > 50%; 21% improved < 50%; 21% did not improve | None 0; 0 |
| 24 1998 | INF-g | Costa’s Index (relative individual response) | 34% improved > 20%; 44% improved < 20%; 22% did not improve Predictors for response: low IgE, low eosinophil cell count at baseline | Not reported Not reported |
Study quality was also very heterogeneous and considered low in many studies included in this review (Table III). Low follow-up rates (< 80% of patients included in the study) were observed in 9 studies (33%), most of which (n = 8; 89%) were RCT (17, 20, 33, 34, 37–39, 41). With respect to internal validity, a low follow-up rate combined with failure to apply intention-to-treat (ITT) analysis is particularly problematic. This combination was present in 3 RCT (17, 38, 41, 44). Less than half of the studies (n = 12; 44%) measured disease severity by means of a validated outcome. The frequent use of unvalidated measurements is likely to cause substantial bias and inaccuracy (45–47). Most RCT did not report on randomization concealment (17, 20, 30, 32–34, 37–39, 41, 42). Randomization concealment was adequate in 4 RCT (31, 35, 36, 40). Blinding procedures were judged adequate in 9 and inadequate in 3 RCT (Table III).
Table III. Summary of study quality
| Ref. / Year | Treatment | Clear case definition | Validated outcome | Follow-up rate > 80% | ITT analysis | Adequate randomization concealment | Adequate blinding procedure |
| 37/1991 | CyA | ● | ○ | ○ | ○ | ○ | ● |
| 38/1994 | CyA | ● | ○ | ○ | ● | ○ | ● |
| 17/1996 | CyA | ● | ○ | ○ | ● | ○ | n.a. |
| 19/1996 | CyA | ○ | ● | ● | n.a. | n.a. | n.a. |
| 18/1997 | CyA | ○ | ● | ○ | n.a. | n.a. | n.a. |
| 20/2000 | CyA | ○ | ● | ○ | ○ | ○ | n.a. |
| 31/2000 | CyA | ● | ○ | ● | ● | ● | ● |
| 21/2000 | CyA | ● | ● | ● | n.a. | n.a. | n.a. |
| 23/2001 | CyA | ● | ● | ● | n.a. | n.a. | n.a. |
| 30/2004 | CyA | ○ | ● | ● | ○ | ○ | ● |
| 22/2001 | CyA | ● | ○ | ● | n.a. | n.a. | n.a. |
| 35/1984 | BMDP | ○ | ○ | ● | ○ | ● | ● |
| 42/1995 | Flunisolide | ● | ○ | ● | ○ | ○ | ○ |
| 36/1993 | INF-γ | ● | ○ | ● | ○ | ● | ○ |
| 25/1993 | INF-γ | ● | ○ | ● | n.a. | n.a. | n.a. |
| 24/1998 | INF-γ | ● | ● | ● | n.a. | n.a. | n.a. |
| 39/2000 | INF-γ | ● | ○ | ○ | ○ | ○ | ○ |
| 26/1998 | IVIG | ○ | ○ | ● | n.a. | n.a. | n.a. |
| 40/2002 | IVIG | ● | ● | ● | ● | ● | n.a. |
| 27/2002 | IVIG | ● | ○ | ● | n.a. | n.a. | n.a. |
| 29/2000 | MMF | ● | ● | ● | n.a. | n.a. | n.a. |
| 28/2001 | MMF | ● | ● | ● | n.a. | n.a. | n.a. |
| 41/2002 | Azathioprine | ● | ● | ○ | ● | ○ | ● |
| 43/2005 | Infliximab | ○ | ● | ● | n.a. | n.a. | n.a. |
| 33/1992 | CHT | ● | ○ | ○ | ○ | ○ | ● |
| 34/1992 | CHT | ○ | ○ | ○ | ○ | ○ | ● |
| 32/1999 | CHT | ● | ○ | ● | ○ | ○ | ● |
●: quality criterion adequately met; ○: quality criterion not adequately met; n.a: not applicable; ITT: intention to treat analysis (44); CyA: cyclosporin A; BMDP: beclomethasonediproprionate; INFγ; interferon-gamma; IVIG: intravenous immunoglobulins; MMF: mycophenolate mofetile; CHT: Chinese herbal therapy.
Because of substantial qualitative heterogeneity in study type, outcome assessment, and study quality we did not pool studies on the same therapeutic agents and did not compare treatments in a meta-analysis.
In the following we will qualitatively summarize the results of the studies included by treatment type.
Cyclosporin A
All 11 studies on CyA showed a decrease in disease activity after treatment, which was superior to placebo in all placebo-controlled RCT (17–23, 30, 31, 37, 38) (Table II). The only study which compared CyA against a different agent was performed by Pacor et al. (30). The authors reported superiority of topical tacrolimus 0.1% twice daily compared with CyA (3 mg/kg). However, due to higher baseline severity in the CyA group, the statistics presented in this paper, i.e. comparison of areas under curves, are inappropriate. After re-analysis of the data we found similar effectiveness of both agents (Table II). Seven studies measured disease activity 6–8 weeks after initiation of CyA treatment. In these studies the mean benefit was consistently a reduction in AE severity of about 50% or more (19, 21, 23, 30, 31, 37, 38). A positive dose-response relationship with 29% vs. 46% mean relative benefit after 2 weeks of treatment with 3 mg/kg vs. 5 mg/kg CyA was observed by Zonneveld et al. (17). The effectiveness of CyA was similar in studies focusing exclusively on children (n = 3) (19, 20, 23) and those including only adult patients (n = 5) (17, 21, 31, 37, 38). Many study protocols permitted individual adjustments to the minimum effective CyA dosage (17, 18, 20, 23, 31). Long-term effectiveness of CyA treatment was evaluated in 3 studies, each of which had a follow-up time of approximately 1 year (17, 18, 20). Mean relative improvement was about 50% in each study. However, with drop-out rates of 62% (18), 35% (17), and 28% (20) and failure to perform an ITT analysis, these results might be explained by emigrative selection bias (48). Harper et al. (20) also studied relapse-rates after discontinuation of CyA treatment. Within 9 months of follow-up a relapse (defined as increase in disease severity to more than 75% of the individual baseline score) was observed in 86% of patients. Withdrawals due to adverse events occurred on average in 0.95% patient months of CyA treatment. In 2 studies no severe adverse events (SAE) were observed (30, 31). No information on the occurrence of SAE was provided in 5 articles (19, 21–23, 38). In the remaining 4 articles a total of 22 SAE occurred, including infections, abdominal pain, acute cholecystitis, and basal cell carcinoma (17, 18, 20, 37).
Systemic glucocorticosteroids
Two small RCT evaluating systemic glucocorticosteroids in severe AE were identified (35, 42). In both studies only children were included. After 4 weeks of treatment with beclomethasone diproprionate (0.8 mg/kg oral + 0.4 mg/kg nasal) mean severity of AE decreased by 22%. One patient was withdrawn because of whooping cough (35). After 2 weeks of treatment with flunisolide (age-adjusted dose, see Table II) mean clinical severity could be reduced by 39%. Within the short observation period of 3 weeks after discontinuation of treatment no relapses (not defined) were observed (42). In both studies no SAEs were observed (35, 42) (Table II). No data was identified for prednisolone, which is the standard systemic glucocorticosteroid used in clinical practice.
Interferon-g
Two RCT and 2 uncontrolled trials were identified on IFN (24, 25, 36, 39). Both RCT included adults and children treated for 12 weeks, did not meet important quality criteria, and did not use a composite score to measure clinical disease severity. IFN was superior to placebo in both RCT (36, 39) (Table II). Jang et al. (39) observed a positive dose-response relationship, with about 50% mean reduction in intensity and extent of AE lesions in the high-dose group (1.5 × 106 IU/m2 body surface area (BSA) 3 times weekly). Hanifin et al. (36) reported a mean decrease in the intensity of AE lesions of about 30% (dosage: 1.5 × 106 IU/m2 BSA/day). In both uncontrolled studies the IFN dosage was tapered off over a treatment period of 6 weeks (24, 25). In the study by Noh & Lee (24), which met all quality criteria, response rates were relatively low. A low serum IgE level was a positive predictor for response.
Intravenous immunoglobulins
Overall, the 3 small studies on IVIG eligible for this review did not show pronounced effectiveness (26, 27, 40). However, some of the patients studied in these trials were resistant not only to topical treatments, but also to systemic steroids and/or AZT (26, 27). Hypertension, haematuria, and transient serum creatinine increase were observed in one patient, serum sickness-like reaction in another patient treated with IVIG (26).
Mycophenolate mofetile
The evidence of the effectiveness of MMF in AE is limited to 2 uncontrolled studies including a total of 20 patients (Table I). After 8 and 12 weeks of treatment a mean decrease in disease activity by 55% and 68%, respectively, was observed (28, 29). One patient was withdrawn due to herpes retinitis, no other SAE were reported (28).
Azathioprine
Only one study on AZT met the eligibility criteria for this review (41). In a double-blind placebo-controlled cross-over RCT Berth-Jones et al. (41) observed a mean reduction in disease activity of 27% after 12 weeks of treatment with 2.5 mg/kg AZT. An ITT analysis was performed, so that the low follow-up rate appears less problematic. Four patients were withdrawn prematurely because of adverse events.
Infliximab
In a small uncontrolled study 9 patients were treated with infliximab 5 mg/kg at weeks 0, 2, and 6. At week 10 the relative individual benefit was more than 50% in only 2 patients, whereas disease activity decreased by less than 30% in 6 patients. One patient dropped out due to a serious infusion reaction (43) (Table II).
Chinese herbal therapy
Three double-blind placebo-controlled cross-over RCT evaluated the efficacy of standardized formulation of 10 herbs (Zemaphyte®, Phytopharm plc,Cambs, UK) (32–34). In these trials no composite severity score was used, so that the results cannot be reliably compared with other studies included in this review. Although the methodology was very similar in these 3 RCT, the results are conflicting: CHT was effective in the 2 studies from the UK, whereas no significant difference from placebo was observed in the study performed in Hong Kong (32–34). In the 2 studies mentioned first, the positive results might be explained by emigrative selection bias due to low follow-up rates and inadequate statistical methods (33, 34, 48).
DISCUSSION
Main findings on specific therapies
To date, CyA is the only systemic agent for which convincing evidence of effectiveness exists in patients with severe AE. All 11 studies we identified consistently showed substantial beneficial effects (17–23, 30, 31, 37, 38). We suggest using CyA for short-term or intermittent long-term therapy in patients resistant to topical anti-inflammatory agents such as glucocorticosteroids and calcineurin inhibitors. Dosages should be adjusted to minimum effective individual levels. Contraindications include hypertension, nephropathy, and history of skin or internal cancer (49–52).
AZT or IFN could be used for short-term treatment in patients who are not eligible for or unresponsive to CyA treatment. For these agents, evidence of the efficacy can be derived from RCT, although only a few patients were analysed in these studies. Compared with CyA, the benefit of AZT and IFN seems to be less pronounced (36, 39, 41). Although only one RCT evaluated its efficacy in patients with AE, AZT is frequently applied in clinical practice (53). AZT increases the risk of squamous cell carcinoma by generating mutagenic oxidative DNA damage (54, 55). Myelotoxicity of AZT is increased in patients with thiopurine methyl transferase (TPMT) deficiency. TPMT-based dosing of AZT seems to reduce toxicity without loss of efficacy (56, 57).
Although systemic glucocorticosteroids are frequently used for short-term therapy of AE in clinical practice there is insufficient evidence from clinical studies (35, 42). Studies including adult patients have not been published at all.
MMF might be a valuable treatment option, but evidence is restricted to 2 small uncontrolled studies (28, 29). From an evidence-based medicine perspective both IVIG and infliximab should be considered only in patients in whom disease activity cannot be sufficiently controlled with other systemic treatment options including CyA, systemic glucocorticosteroids, AZT, and IFN.
The results of the 3 RCT on CHT are conflicting. The 2 trials showing positive effects of CHT did not meet critically important methodological criteria: the end-points used are unvalidated and constructed qualitatively differently from the end-points applied in the majority of other studies reviewed (32–34, 48). Adequate comparison of the effectiveness of CHT and other agents is impossible. Zemaphyte® is a standardized preparation of therapeutic herbs for the treatment of AE. This is consistent with the concept of Western medicine: to treat certain diseases with certain substances. By contrast, traditional Chinese medicine prefers an individualized polypharmacology approach and emphasizes the importance of treating the whole individual rather than a certain diagnosis. Therefore, advocates of traditional Chinese medicine argue that this conceptual difference explains the failure of efficacy of Zemaphyte® in many patients (32). Reports of severe toxicity of CHT including fatal hepatitis highlight the significance of regularly monitoring patients treated with traditional Chinese medicine (58–60). Further well-designed, larger scale trials are required, but Zemaphyte® is no longer available.
Study quality
A major concern is that important quality criteria were not met in a high proportion of studies included in this review. High drop-out rates, imprecise case definition, inadequate statistical methods, inadequate randomization concealment and/or blinding procedures, and unvalidated outcome measurements are well-known threats to internal validity (48). The use of many different, in many cases unvalidated, outcome assessments for disease severity was a major source of heterogeneity. This was one reason why meta-analysis could not be performed.
Limitations of this review
All systemic treatment options discussed are known to be associated with potentially severe ADR (12, 49, 51, 61, 62). Small short-term clinical studies like most of the ones discussed in this review are not appropriate to evaluate long-term safety or rare ADRs. We used withdrawals due to ADRs and SAEs as surrogate parameters for safety. Particular safety concerns were not revealed. However, the reporting quality of adverse events was inadequate in a high percentage of studies. It is questionable whether all ADRs were disclosed. Therefore, it was not possible to compare the benefit-to-risk ratio of the different agents reviewed. Because of potentially SAEs, systemic remedies should be restricted to patients who do not adequately respond to both topical therapies (first-line therapy for AE) and phototherapy (second-line therapy) (10, 11, 63–66). When administering systemic treatments in AE 2 different goals may be pursued: to induce or to maintain remission. Efficacy is typically defined as a drug’s potential to decrease disease severity, i.e. its potential to induce remission. Because most studies focused on this aspect, our recommendations primarily relate to induction of remission in severe AE.
Research recommendations
It is critically necessary to standardize outcome assessments used in clinical investigation on AE. A core set of outcomes for defined settings (e.g. RCT, clinical record keeping) should be identified, e.g. using consensus methods (67). A standardization of outcome methodology would enable us to approach many clinically important, yet unanswered, questions, e.g. the additional benefit of topical therapies and quantitative comparisons of the effectiveness of different treatment options.
To clarify the relative importance of systemic glucocorticosteroids, comparative clinical studies, e.g. against CyA, should be performed. In addition to efficacy this research should focus on relapse rates after discontinuation of treatment, tolerability, additional benefits of topical treatments, dosing regimens with optimal benefit-to-risk ratio, and possible predictors of treatment success. Additionally, studies on topical vs. systemic steroids are encouraged.
Although the data on efficacy is convincing, CyA may cause kidney damage and other ADR when used as a long-term treatment. Therefore, we should evaluate other treatment options with better safety profiles in long-term RCT. Leflunomide might be such a therapeutic alternative, but larger scale trials are required (68).
Because most studies included in this review looked only at induction of remission, long-term studies on remission maintenance are encouraged.
Implications for clinical practice
Current guidelines on the treatment of patients with AE do not always reflect published evidence (10). The International Consensus Conference on Atopic Dermatitis II (2003) suggested using systemic steroids, CyA, methotrexate, or AZT for patients whose disease is resistant to topical anti-inflammatory agents (10). Although the evidence is very different for these treatment options in terms of quality, quantity and results, the consensus did not provide an algorithm for the preference of systemic treatments for AE. Based on the results of this systematic review, treatment guidelines should be updated appropriately.
Conflict of interest: No conflict of interest to declare,
References
1. Williams HC. Clinical practice. Atopic dermatitis. N Engl J Med 2005; 352: 2314–2324.
2. Williams H, Robertson C, Stewart A, Ait-Khaled N, Anabwani G, Anderson R, et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. J Allergy Clin Immunol 1999; 103: 125–138.
3. Rystedt I. Long-term follow-up in atopic dermatitis. Acta Derm Venereol Suppl 1985; 114: 117–120.
4. Lammintausta K, Kalimo K, Raitala R, Forsten Y. Prognosis of atopic dermatitis. A prospective study in early adulthood. Int J Dermatol 1991; 30: 563–568.
5. Carroll CL, Balkrishnan R, Feldman SR, Fleischer AB, Jr, Manuel JC. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol 2005; 22: 192–199.
6. Kemp AS. Cost of illness of atopic dermatitis in children: a societal perspective. Pharmacoeconomics 2003; 21: 105–113.
7. Emerson RM, Williams HC, Allen BR. Severity distribution of atopic dermatitis in the community and its relationship to secondary referral. Br J Dermatol 1998; 139: 73–76.
8. Chamlin SL, Frieden IJ, Williams ML, Chren MM. Effects of atopic dermatitis on young American children and their families. Pediatrics 2004; 114: 607–611.
9. Fivenson D, Arnold RJ, Kaniecki DJ, Cohen JL, Frech F, Finlay AY. The effect of atopic dermatitis on total burden of illness and quality of life on adults and children in a large managed care organization. J Manag Care Pharm 2002; 8: 333–342.
10. Ellis C, Luger T, Abeck D, Allen R, Graham-Brown RA, De Prost Y, et al. International Consensus Conference on Atopic Dermatitis II (ICCAD II): clinical update and current treatment strategies. Br J Dermatol 2003; 148 suppl 63: 3–10.
11. Hanifin JM, Cooper KD, Ho VC, Kang S, Krafchik BR, Margolis DJ, et al. Guidelines of care for atopic dermatitis, developed in accordance with the American Academy of Dermatology (AAD)/American Academy of Dermatology Association “Administrative Regulations for Evidence-Based Clinical Practice Guidelines”. J Am Acad Dermatol 2004; 50: 391–404.
12. Heratizadeh A, Breuer K, Kapp A, Werfel T. Systemic therapy of atopic dermatitis. Hautarzt 2003; 54: 937–945.
13. Schneider LC, Baz Z, Zarcone C, Zurakowski D. Long-term therapy with recombinant interferon-gamma (rIFN-gamma) for atopic dermatitis. Ann Allergy Asthma Immunol 1998; 80: 263–268.
14. Salek MS, Finlay AY, Luscombe DK, Allen BR, Berth-Jones J, Camp RD, et al. Cyclosporin greatly improves the quality of life of adults with severe atopic dermatitis. A randomized, double-blind, placebo-controlled trial. Br J Dermatol 1993; 129: 422–430.
15. Sheehan MP, Stevens H, Ostlere LS, Atherton DJ, Brostoff J, Rustin MH. Follow-up of adult patients with atopic eczema treated with Chinese herbal therapy for 1 year. Clin Exp Dermatol 1995; 20: 136–140.
16. Stevens SR, Hanifin JM, Hamilton T, Tofte SJ, Cooper KD. Long-term effectiveness and safety of recombinant human interferon gamma therapy for atopic dermatitis despite unchanged serum IgE levels. Arch Dermatol 1998; 134: 799–804.
17. Zonneveld IM, de Rie MA, Beljaards RC, Van Der Rhee HJ, Wuite J, Zeegelaar J, et al. The long-term safety and efficacy of cyclosporin in severe refractory atopic dermatitis: a comparison of two dosage regimens. Br J Dermatol 1996; 135 suppl 48: 15–20.
18. Berth-Jones J, Graham-Brown RA, Marks R, Camp RD, English JS, Freeman K, et al. Long-term efficacy and safety of cyclosporin in severe adult atopic dermatitis. Br J Dermatol 1997; 136: 76–81.
19. Berth-Jones J, Finlay AY, Zaki I, Tan B, Goodyear H, Lewis-Jones S, et al. Cyclosporine in severe childhood atopic dermatitis: a multicenter study. J Am Acad Dermatol 1996; 34: 1016–1021.
20. Harper JI, Ahmed I, Barclay G, Lacour M, Hoeger P, Cork MJ, et al. Cyclosporin for severe childhood atopic dermatitis: short course versus continuous therapy. Br J Dermatol 2000; 142: 52–58.
21. Caproni M, Salvatore E, Cardinali C, Brazzini B, Fabbri P. Soluble CD30 and cyclosporine in severe atopic dermatitis. Int Arch Allergy Immunol 2000; 121: 324–328.
22. Pacor ML, Biasi D, Di Lorenzo G, Carletto A, Corrocher R. Cyclosporin in atopic dermatitis. Recenti Prog Med 2001; 92: 390–391.
23. Bunikowski R, Staab D, Kussebi F, Brautigam M, Weidinger G, Renz H, et al. Low-dose cyclosporin A microemulsion in children with severe atopic dermatitis: clinical and immunological effects. Pediatr Allergy Immunol 2001; 12: 216–223.
24. Noh GW, Lee KY. Blood eosinophils and serum IgE as predictors for prognosis of interferon-gamma therapy in atopic dermatitis. Allergy 1998; 53: 1202–1207.
25. Reinhold U, Kukel S, Brzoska J, Kreysel HW. Systemic interferon gamma treatment in severe atopic dermatitis. J Am Acad Dermatol 1993; 29: 58–63.
26. Wakim M, Alazard M, Yajima A, Speights D, Saxon A, Stiehm ER. High dose intravenous immunoglobulin in atopic dermatitis and hyper-IgE syndrome. Ann Allergy Asthma Immunol 1998; 81: 153–158.
27. Jolles S, Sewell C, Webster D, Ryan A, Heelan B, Waite A, Rustin M. Adjunctive high-dose intravenous immunoglobulin treatment for resistant atopic dermatitis: efficacy and effects on intracellular cytokine levels and CD4 counts. Acta Derm Venereol 2003; 83: 433–437.
28. Grundmann-Kollmann M, Podda M, Ochsendorf F, Boehncke WH, Kaufmann R, Zollner TM. Mycophenolate mofetil is effective in the treatment of atopic dermatitis. Arch Dermatol 2001; 137: 870–873.
29. Neuber K, Schwartz I, Itschert G, Dieck AT. Treatment of atopic eczema with oral mycophenolate mofetil. Br J Dermatol 2000; 143: 385–391.
30. Pacor ML, Di Lorenzo G, Martinelli N, Mansueto P, Rini GB, Corrocher R. Comparing tacrolimus ointment and oral cyclosporine in adult patients affected by atopic dermatitis: a randomized study. Clin Exp Allergy 2004; 34: 639–645.
31. Czech W, Brautigam M, Weidinger G, Schopf E. A body-weight-independent dosing regimen of cyclosporine microemulsion is effective in severe atopic dermatitis and improves the quality of life. J Am Acad Dermatol 2000; 42: 653–659.
32. Fung AY, Look PC, Chong LY, But PP, Wong E. A controlled trial of traditional Chinese herbal medicine in Chinese patients with recalcitrant atopic dermatitis. Int J Dermatol 1999; 38: 387–392.
33. Sheehan MP, Rustin MH, Atherton DJ, Buckley C, Harris DW, Brostoff J, et al. Efficacy of traditional Chinese herbal therapy in adult atopic dermatitis. Lancet 1992; 340: 13–17.
34. Sheehan MP, Atherton DJ. A controlled trial of traditional Chinese medicinal plants in widespread non-exudative atopic eczema. Br J Dermatol 1992; 126: 179–184.
35. Heddle RJ, Soothill JF, Bulpitt CJ, Atherton DJ. Combined oral and nasal beclomethasone diproprionate in children with atopic eczema: a randomised controlled trial. Br Med J (Clin Res Ed) 1984; 289: 651–654.
36. Hanifin JM, Schneider LC, Leung DY, Ellis CN, Jaffe HS, Izu AE, et al. Recombinant interferon gamma therapy for atopic dermatitis. J Am Acad Dermatol 1993; 28: 189–197.
37. Sowden JM, Berth-Jones J, Ross JS, Motley RJ, Marks R, Finlay AY, et al. Double-blind, controlled, crossover study of cyclosporin in adults with severe refractory atopic dermatitis. Lancet 1991; 338: 137–140.
38. van Joost T, Heule F, Korstanje M, van den Broek MJ, Stenveld HJ, van Vloten WA. Cyclosporin in atopic dermatitis: a multicentre placebo-controlled study. Br J Dermatol 1994; 130: 634–640.
39. Jang IG, Yang JK, Lee HJ, Yi JY, Kim HO, Kim CW, Kim TY. Clinical improvement and immunohistochemical findings in severe atopic dermatitis treated with interferon gamma. J Am Acad Dermatol 2000; 42: 1033–1040.
40. Paul C, Lahfa M, Bachelez H, Chevret S, Dubertret L. A randomized controlled evaluator-blinded trial of intravenous immunoglobulin in adults with severe atopic dermatitis. Br J Dermatol 2002; 147: 518–522.
41. Berth-Jones J, Takwale A, Tan E, Barclay G, Agarwal S, Ahmed I, et al. Azathioprine in severe adult atopic dermatitis: a double-blind, placebo-controlled, crossover trial. Br J Dermatol 2002; 147: 324–330.
42. La Rosa M, Musarra I, Ranno C, Maiello N, Negri L, Del Giudice Jr MM, et al. A randomized, double-blind, placebo-controlled crossover trial of systemic flunisolide in the treatment of children with severe atopic dermatitis. Curr Therap Res 1995; 56: 720–726.
43. Jacobi A, Antoni C, Manger B, Schuler G, Hertl M. Infliximab in the treatment of moderate to severe atopic dermatitis. J Am Acad Dermatol 2005; 52: 522–526.
44. Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 1999; 319: 670–674.
45. Chren MM. Giving “scale” new meaning in dermatology: measurement matters. Arch Dermatol 2000; 136: 788–790.
46. Charman C, Chambers C, Williams H. Measuring atopic dermatitis severity in randomized controlled clinical trials: what exactly are we measuring? J Invest Dermatol 2003; 120: 932–941.
47. Charman C, Williams H. Outcome measures of disease severity in atopic eczema. Arch Dermatol 2000; 136: 763–769.
48. Szklo M, Nieto J, editors. Epidemiology beyond the basics. Sidbury, MA: Jones and Barlett Publishers, 2005: p. 205–208.
49. Behnam SM, Behnam SE, Koo JY. Review of cyclosporine immunosuppressive safety data in dermatology patients after two decades of use. J Drugs Dermatol 2005; 4: 189–194.
50. Marcil I, Stern RS. Squamous-cell cancer of the skin in patients given PUVA and ciclosporin: nested cohort crossover study. Lancet 2001; 358: 1042–1045.
51. Paul CF, Ho VC, McGeown C, Christophers E, Schmidtmann B, Guillaume JC, et al. Risk of malignancies in psoriasis patients treated with cyclosporine: a 5 y cohort study. J Invest Dermatol 2003; 120: 211–216.
52. Tugwell P, Baker P. Guidelines for the use of cyclosporine in rheumatoid arthritis. Clin Rheumatol 1995; 14 suppl 2: 37–41.
53. Tan BB, Lear JT, Gawkrodger DJ, English JS. Azathioprine in dermatology: a survey of current practice in the UK. Br J Dermatol 1997; 136: 351–355.
54. Jensen P, Hansen S, Moller B, Leivestad T, Pfeffer P, Geiran O, et al. Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J Am Acad Dermatol 1999; 40: 177–186.
55. O’Donovan P, Perrett CM, Zhang X, Montaner B, Xu YZ, Harwood CA, et al. Azathioprine and UVA light generate mutagenic oxidative DNA damage. Science 2005; 309: 1871–1874.
56. Meggitt SJ, Gray JC, Reynolds NJ. Azathioprine dosed by thiopurine methyltransferase activity for moderate-to-severe atopic eczema: a double-blind, randomised controlled trial. Lancet 2006; 367: 839–846.
57. Sies C, Florkowski C, George P, Gearry R, Barclay M, Harraway J, et al. Measurement of thiopurine methyl transferase activity guides dose-initiation and prevents toxicity from azathioprine. N Z Med J 2005; 118: 1324.
58. Graham-Brown R. Toxicity of Chinese herbal remedies. Lancet 1992; 340: 673–674.
59. MacGregor FB, Abernethy VE, Dahabra S, Cobden I, Hayes PC. Hepatotoxicity of herbal remedies. BMJ 1989; 299: 1156–1157.
60. Mostefa-Kara N, Pauwels A, Pines E, Biour M, Levy VG. Fatal hepatitis after herbal tea. Lancet 1992; 340: 674.
61. Dutz JP, Ho VC. Immunosuppressive agents in dermatology. An update. Dermatol Clin 1998; 16: 235–251.
62. Anstey AV, Wakelin S, Reynolds NJ. Guidelines for prescribing azathioprine in dermatology. Br J Dermatol 2004; 151: 1123–1132.
63. Thestrup-Pedersen K. Treatment principles of atopic dermatitis. J Eur Acad Dermatol Venereol 2002; 16: 1–9.
64. Ravenscroft JC, Thomas KS, Williams HC. Current management of atopic eczema. Practitioner 2002; 246: 690–695.
65. Abeck D, Strom K. Optimal management of atopic dermatitis. Am J Clin Dermatol 2000; 1: 41–46.
66. Krutmann J. Phototherapy for atopic dermatitis. Clin Exp Dermatol 2000; 25: 552–558.
67. Jones J, Hunter D. Consensus methods for medical and health services research. BMJ 1995; 311: 376–380.
68. Schmitt J, Wozel G, Pfeiffer C. Leflunomide as a novel treatment option in severe atopic dermatitis. Br J Dermatol 2004; 150: 1182–1185.
69. Berth-Jones J. Six area, six sign atopic dermatitis (SASSAD) severity score: a simple system for monitoring disease activity in atopic dermatitis. Br J Dermatol 1996; 135 suppl 48: 25–30.
70. Costa C, Rilliet A, Nicolet M, Saurat JH. Scoring atopic dermatitis: the simpler the better? Acta Derm Venereol 1989; 69: 41–45.
71. Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis Dermatology 1993; 186: 23–31.
72. Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol 2001; 10: 11–18.
Table II contd.
Table I. Characteristics of studies included in the systematic review
| Ref. Year | Study design | Country Number enrolled (Age range years) | Inclusion criteria regarding disease severity | Drug Duration of active treatment | Initial dose, comparator (if applicable) | Dose adjustments | Concurrent treatment | ||
| 37 1991 | d-b RCT | UK n = 33 (17–56) | Inadequately controlled by conventional therapies | CyA 8 weeks | 5 mg/kg BW vs. placebo | None | Topical steroids | ||
| 38 1994 | d-b RCT | Netherlands n = 46 (17–68) | Resistant to conventional therapies | CyA 6 weeks | 5 mg/kg BW vs. placebo | None | Antihistamines | ||
| 17 1996 | open RCT | Netherlands n = 78 (18–70) | Resistant to conventional therapy and/or significantly disabling AE | CyA 1 year | 3 mg/kg BW vs. 5 mg/kg BW | After 2 weeks stepwise adjustment to minimum effective dose | Topical steroids, antibiotics, antihistamines | ||
| 19 1996 | open u-c study | UK n = 27 (2–16) | Refractory to topical steroids | CyA 6 weeks | 5 mg/kg BW | None | Topical steroids, antihistamines | ||
| 18 1997 | open u-c study | UK n = 100 (≥12) | Disabling AE, inadequately controlled by topical steroids | CyA 48 weeks | 2.5 mg/kg BW | After 8 weeks stepwise adjustment to minimum effective dose | Topical steroids, antihistamines | ||
| 20 2000 | open RCT | UK n = 43 (2–16 ) | Refractory to topical steroids | CyA 1 year | 5 mg/kg BW; 12 weeks short courses vs. 1 year continuous therapy | After 4 weeks stepwise adjustment to minimum effective dose | Topical steroids | ||
| 31 2000 | d-b RCT | Germany n = 106 (≥18) | Refractory to conventional therapies and BSA 30% or more | CyA 8 weeks | 150 mg vs. 300 mg | After 2 weeks stepwise adjustment to minimum effective dose | Topical steroids, antihistamines | ||
| 21 2000 | open u-c study | Italy n = 10 (17–45) | Resistant to conventional therapies | CyA 6 weeks | 5 mg/kg BW | None | Not reported | ||
| 23 2001 | open u-c study | Germany n = 10 (1–15) | SCORAD > 50 and refractory to topical steroids | CyA 8 weeks | 2.5 mg/kg BW | After 2 weeks stepwise adjustment to minimum effective dose | Topical steroids | ||
| 22 2001 | open u-c study | Italy n = 15; 35.5 (median) | Resistant to conventional therapies | CyA 8 weeks | 5 mg/kg BW | Not reported | Not reported | ||
| 30 2004 | d-b RCT | Italy n = 30 (13–45) | Inadequately controlled by topical steroids | CyA 6 weeks | 3 mg/kg BW vs. topical tacrolimus 0.1% | None | Antihistamines | ||
| 35 1984 | d-b RCT | UK n = 27 (3–14) | Inadequately controlled by conventional therapies | Beclomethasone- diproprionate 4 weeks | 0.8 mg/day oral + 0.4 mg/day nasal | None | Topical steroids, antihistamines | ||
| 42 1995 | d-b RCT | Italy n = 20 (2–6 ) | Inadequately controlled by topical therapies | Flunisolide 2 weeks | 0.64 mg/day (age 2 years) 1.2 mg/day (age 3–6 years) | None | Antihistamines | ||
| 36 1993 | d-b RCT | USA n = 83 (2–65) | Inadequately controlled by conventional therapies | INF-g 12 weeks | 1.5 × 106 IU/m2/day vs. placebo | None | Systemic and topical steroids, antihistamines | ||
| 25 1993 | open u-c study | Germany n = 14 (22–33) | Inadequately controlled by topical steroids | INF-g 6 weeks | 5 × 2 × 106 IU in 1st week 3 × 2× 106 IU in week 2–4 2 × 2 × 106 IU in week 5–6 | None | None | ||
| Ref. Year | Study design | Country Number enrolled Age range (years) | Inclusion criteria regarding disease severity | Drug Duration of active treatment | Initial dose, comparator (if applicable) | Dose adjustments | Concurrent treatment | ||
| 24 1998 | open u-c study | Korea n = 68 (not stated) | Inadequately controlled by conventional therapies | INF-g 6 weeks | 5 × 106 IU/m2 in 1st week 3 × 106 IU/m2 in week 2–4 2 × 106 IU/m2 in week 5–6 | None | None | ||
| 39 2000 | d-b RCT | Korea n = 51 (≥15) | Inadequately controlled by conventional therapies, BSA > = 20% | INF-g 12 weeks | 1.5 × 106 IU/m2 3 × / week vs. 0.5 × 106 IU/m2 3 × weekly vs. placebo | None | None | ||
| 26 1998 | open u-c study | USA n = 9 (7–64) | Inadequately controlled by conventional therapies | IVIG 7 months | 2 g/kg BW / month | None | Systemic and topical steroids, antihistamines | ||
| 40 2002 | e-b RCT | France n = 10 (21–38 ) | SCORAD > 50 and inadequately controlled by conventional therapies | IVIG 1 cycle (evaluation at day 30) | 2 g/kg within 2 days (immediate or delayed treatment (at day 31)) | None | Topical steroids | ||
| 27 2002 | open u-c study | UK n = 6 (≥18) | Inadequately controlled by conventional therapies | IVIG 6 months | 2 g/kg BW / month | None | Systemic/topical steroids, azathiorine antihistamines, | ||
| 29 2000 | open u-c study | Germany n = 10 (29–47) | Inadequately controlled by conventional therapies | MMF 12 weeks | 1g/day in week 1 2g/day in week 2–12 | None | Topical steroids | ||
| 28 2001 | open u-c study | Germany n = 10 (19–66) | Inadequately controlled by conventional therapies | MMF 8 weeks | 2g/day in week 1–4 1 g/day in week 5–8 | None | Topical steroids in week 1–2; none in week 3–8 | ||
| 41 2002 | d-b RCT | UK n = 37 (17–73) | Inadequately controlled by topical steroids | Azathioprine 12 weeks | 2.5 mg/kg BW vs. placebo | None | Topical steroids | ||
| 43 2005 | open u-c study | Germany n = 9 (19–61) | Resistant to conventional therapies | Infliximab 10 weeks (primary end-point) | 5 mg/kg BW at weeks 0, 2, and 6 | None | Topical steroids | ||
| 33 1992 | d-b RCT | UK n = 40 (19–57) | Extensive (> 20% BSA) and refractory disease | CHT 8 weeks | Standardized formulation of 10 herbs (Zemaphyte) vs. placebo | None | Topical steroids | ||
| 34 1992 | d-b RCT | UK n = 47 (1–18) | Resistant to conventional therapies | CHT 8 weeks | Standardized formulation of 10 herbs (Zemaphyte) vs. placebo | None | None | ||
| 32 1999 | d-b RCT | Hong Kong n = 40 (7–50) | Inadequately controlled by topical treatment | CHT 8 weeks | Standardized formulation of 10 herbs (Zemaphyte) vs. placebo | None | Topical steroids |
AE: atopic eczema; BSA: body surface area; BW: body+weight; CHT: Chinese herbal therapy; c-o: cross-over; d-b: double-blind; u-c: uncontrolled; e-b: evaluator-blinded; CyA: cyklosporin A; INF-g: interferon-gamma; IVIG: intravenous immunoglobulim; IU: international units; MMF: mycophenolate mofetile; RTC: randomized controlled trial; SCORAD: Scoring Atopic Dermatitis Index (70).
