Sir,
Argyria is a cutaneous discoloration caused by intake of silver or various silver compounds. Because silver is not absorbed through normal skin, it is assumed that argyria is caused by the absorption of silver via ingestion, mucosa, or implant by medical instruments that include silver. There have been some cases reported of argyria caused by silver used in medical applications (1, 2). Case reports have also been described in which argyria was caused by habitual use of a large quantity of a mouthwash (Jintan) in Japan (3). In addition, some cases have been reported of argyria being induced by silver in a particular type of sugar (4), and colloidal silver protein as a food supplement (5).
In this report we describe a case of argyria caused by the silver protein used in medical applications.
Case Report
A 69-year-old woman was referred to our outpatient clinic because of a 2-year history of slate gray-coloured pigmentation in her face and dorsal hands. She had undergone topical treatment with silver protein (Protein Gin®) five times a week for pharyngitis for more than 5 years by an otorhinologist.
Physical examination revealed bronze-coloured pigmentation in the face (Fig. 1a), dorsal hands, nail bed (Fig. 1b) and limbs. Pigment freckles were not seen on the lips and the mouth, and the non-exposed parts of the limbs and trunk were normal. We suspected argyria, Wilson’s disease or Addison’s disease. The laboratory tests were within normal ranges. Her serum copper level was 106 µg/dl (normal range 68–128 µg/dl), and the serum ceruloplasmin was 20.5 mg/dl (normal range 14–34 mg/dl). A skin biopsy of the dorsal hand revealed an increase in melanin granules in the epidermal basal layer. Black particles were seen between collagen bundles and around skin appendages in the dermis. Berlin-blue stain was negative. Electron microscopic examination disclosed electron-dense granules scattered around collagen fibres and fibroblasts in the dermis (Fig. 2).
Fig. 1. A 69-year-old Japanese woman. Slate gray-coloured pigmentation was seen in (a) the face and (b) dorsal hands and finger nails.
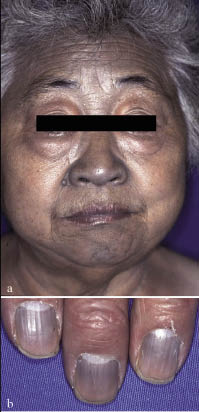

Fig. 2. Electron microscopy shows electron dense particles between collagen fibres in the dermis (arrows).
X-ray microanalysis revealed that granulate materials contained silver, selenium and sulphur. In particular, a high peak of silver was seen (Fig. 3). In light of these findings, we diagnosed this case as generalized argyria.
Because the patient had no other history of contact with silver other than silver protein used as an antiseptic, we concluded this to be the causative agent. We consulted her to keep away from sunlight, then she improved fairly.

Fig. 3. Spot X-ray microanalytical spectrograph. Note the prominent silver peak (Ag).
Discussion
Argyria results from deposition of silver in the skin. In our case it is supposed that silver protein used for the treatment of pharyngitis caused a generalized argyria. Some similar cases have been previously reported (6, 7). As observed in our case, argyria pigmentation is usually recognized only in sun-exposed areas. It has been proposed that elemental silver is reduced to silver sulphide and selenide in the skin, catalysed by sunlight in a process similar to photography (5), which causes the discolouration.
A confirmation of the existence of silver is required for the definite diagnosis of argyria by skin biopsy, X-ray microanalysis assay, atomic absorptiometry, and processing by silver solution.
At histopathological investigations, the existence of silver can be recognized as black granules around sweat glands, sebaceous glands, connective tissues, and basement membranes by light and electron microscopy. According to the previous reports, silver is generally detected in the granular material in X-ray microanalysis assay.
Potassium iodide, metal chelating medicine, glutathione administered internally, and injection of brine are used for the treatment of argyria; however, so far none of these treatments have resulted in any significant improvement. Avoiding the causative agent is the most important aspect of treatment (8). Although our case just kept away from sunlight, she showed some improvement, and no aggravation was observed after ceasing silver protein for antiseptic.
referenceS
1. Fung MC, Bowen DL. Silver products for medical indications: risk-benefit assessment. J Toxicol Clin Toxicol 1996; 34: 119–126.
2. Payne CM, Bladin C, Colchester AC, Bland J, Lapworth R, Lane D. Argyria from excessive use of topical silver sulphadiazine. Lancet 1992; 340: 126.
3. Ohbo Y, Fukuzako H, Takeuchi K, Takigawa M. Argyria and convulsive seizures caused by ingestion of silver in a patient with schizophrenia. Psychiatry Clin Neurosci 1996; 50: 89–90.
4. Hanada K, Hashimoto I, Kon A, Kida K, Mita R. Silver in sugar particles and systemic argyria. Lancet 1998; 351: 960.
5. White JML, Powell AM, Brady K, Russell-Jones R. Severe generalized argyria secondary to ingestion of colloidal silver protein. Clin Exp Dermatol 2003; 28: 254–256.
6. Marshall JP, Schneider RP. Systemic argyria secondary to topical silver nitrate. Arch Dermatol 1977; 113: 1077–1079.
7. Akimov VN. Argyria caused by uncontrolled use of silver protein in vasomotor rhinitis. Vestn Otorinolaringol 1980; 6: 77–78.
8. Shelley WB, Shelley ED, Burmeister V. Argyria: the intradermal “photograph”, a manifestation of passive photosensitivity. J Am Acad Dermatol 1987; 16: 211–217.
Noriyasu Sakai1, Mikako Aoki1, Shichiro Miyazawa2, Masahiko Akita1, Shinichiro Takezaki1 and Seiji Kawana1
1Department of Dermatology, Nippon Medical School, Tokyo, and 2Department of Electron Microscopy, Kitasato University School of Medicine, Kanagawa, Japan. E-mail: nori@yg8.so-net.ne.jp
Accepted September 4, 2006.
