The burdens of childhood eczema are many and some can be assessed with quality of life (QoL) questionnaires. Seventy-eight Swedish children with mild-to-severe eczema (“atopic dermatitis”, prurigo Besnier), fulfilling established diagnostic criteria, were investigated for the effect of eczema on QoL. This was measured with validated questionnaires: the Infants’ Dermatitis Quality of Life Index (IDQOL), the Children’s Dermatology Life Quality Index (CDLQI), and the Dermatitis Family Impact Questionnaire (DFI). The study also included scoring of eczema severity. The median score was 7.0 (range 1–18) for IDQOL, 6.0 (range 2–18) for the CDLQI, and 8.0 (range 0–27) for DFI. There was no significant difference in scores between boys and girls. The DFI scores were higher for younger than for older children, and also higher for those with both eczema and asthma, food allergy/intolerance, allergic rhinoconjunctivitis or urticaria. The QoL scores correlated significantly with the Rajka & Langeland score, but not with objective SCORAD. The outcome of the QoL instruments in this study clearly demonstrates that childhood eczema affects the children’s and their families’ QoL. QoL data offers a patient-oriented outcome measure of importance for understanding the patients’ and their families’ situation. Such information can also be used in intervention studies and in the allocation of healthcare resources to eczema care. Key words: atopic dermatitis; health-related quality of life; Children’s Dermatology Life Quality Index (CDLQI); Dermatitis Family Impact Questionnaire (DFI); Infants’ Dermatitis Quality of Life Index (IDQOL); SCORAD; severity.
(Accepted December 18, 2006.)
Acta Derm Venereol 2007; 87: 345–349.
Agneta Gånemo, RN, PhD, Department of Dermatology, Malmö University Hospital MAS, SE-205 02 Malmö, Sweden. E-mail: agneta.ganemo@skane.se
Eczema (1), still called atopic eczema/atopic dermatitis/prurigo Besnier by many, is a chronic inflammatory pruritic skin disorder affecting approximately 20% of Swedish children (2, 3). The many burdens of childhood eczema range from mild to severe: eczema can interfere with the child’s and their family’s everyday life through, for example, itch, pain, sleep loss, impaired emotional and social contacts, financial costs and time-consuming treatments.
There are many ways to measure disease activity in eczema (4). Assessments of itching (5), sleep loss (6) or quality of life (QoL) (7) offer patient-oriented measures, rating symptoms, signs or feelings that are important to patients, to their families (8) and to healthcare providers. During the 1990s, questionnaires were developed and validated for QoL measurement in children with skin disorders (Children’s Dermatology Life Quality Index (CDLQI) (9)), infants with eczema (Infants’ Dermatitis Quality of Life Index (IDQOL) (10)), and families having a child with eczema (Dermatitis Family Impact Questionnaire (DFI) (11)). These questionnaires, which have been translated into several languages (12), cover, for example, symptoms and feelings/mood, disease severity, feeding, dressing/undressing, housework, leisure, school or holidays, personal relationships, sleep, expenditure and treatment.
The aim of the present study was to adapt and test Swedish versions of QoL instruments and to explore and assess QoL in Swedish children with eczema and the impact of eczema on their families. Such data is required in order to understand the patients’ and families’ situation, and can be used in intervention studies and to support arguments for the allocation of healthcare resources to eczema care.
MATERIALS AND METHODS
Subjects
Subjects with a working diagnosis of eczema were recruited from new referrals or follow-up visits at the departments of dermatology in two Swedish university hospitals (Malmö University Hospital MAS and Karolinska University Hospital Solna). Inclusion criteria were diagnosis according to the UK Working Party’s Diagnostic Criteria (13, 14), age between 2 and 16 years, command of the Swedish language, and oral and written informed consent. To study age-specific QoL patterns, the children were recruited from three different age groups (2–4, 5–8 and 9–16 years). The Regional Ethical Review Board in Lund approved the study (number 333–2004), which was performed in separate research outpatient clinics from November 2004 through March 2006.
Questionnaires
QoL was assessed with established and validated questionnaires. The IDQOL (10) was used for children between 2 and 4 years of age, while the CDLQI (9) was used for children age 5 years or older. In addition, the parent(s) answered the DFI (11). The questionnaires covered the preceding 7 days and had 10 questions, each scoring 0–3, giving a maximum score per questionnaire of 30; the higher the score, the more QoL is impaired. In addition, the IDQOL includes a question scored separately from the QoL index, dealing with dermatitis severity (0–4) as perceived by the carer.
Since the DFI was not available in Swedish, three of the investigators (AG, ML and CFW) translated it separately from English to Swedish, and then discussed and agreed on the best final single translation. Next, two professional translators back-translated the Swedish questionnaire into English, with a result almost identical to the original form, requiring only a minor adjustment in one question. A pilot study of 20 parents with children with eczema confirmed that the DFI questionnaire was comprehensible and clear. Subsequently, the English copyright holder was notified.
Examination
At the visit, which lasted 1.5 h, the child and its parent(s) first visited a dermatologist (ÅS, CFW) and then a dermatology research nurse (AG). The child’s present and past medical history was obtained using a structured questionnaire. The dermatologist examined the child and verified that the child’s skin disorder met the diagnostic criteria. The dermatology research nurse scored eczema severity using Rajka & Langeland (15) and SCORAD scoring (16, 17). The Rajka & Langeland score pays attention to the course during the previous year as well as to present eczema extent and itch. Its scores range between 0 and 9 (mild 3–4, moderate 4.5–7.5, severe 8–9). The quality of the SCORAD scoring had been evaluated in a pilot study that showed good agreement (Fleiss’ kappa, 0.69, p = 3 × 10–7) between the research nurse and the dermatologists. Both objective SCORAD (investigator’s rating of extent and intensity; possible range 0–83; mild eczema score 0–14, moderate eczema 15–40, severe eczema >40) (17) and total SCORAD (investigator’s rating of extent and intensity and patient’s/parent’s rating of pruritus and sleep loss; possible range 0–103) (16, 17) were calculated. After the scoring of the eczema severity, the QoL questionnaires were distributed and completed. For children between 2 and 4 years of age, the parent(s) answered the IDQOL and DFI questionnaires. For children aged 5 years or older, the CDLQI was completed by the research nurse in interaction with the child alone (i.e. the parent(s) left the room), whereas the DFI was completed by the parent(s) separately.
Statistics
Statistical analyses were made with SPSS 14.0 Windows. The Wilcoxon signed-rank test was used for comparisons between dependent samples, while the Mann-Whitney U test was used for comparisons between independent samples. Spearman’s rank order correlation coefficient was computed as measure of association. The tests were two-tailed.
RESULTS
Subjects
Of 82 children invited to participate in the study, 78 (38 males, 40 females) met the inclusion criteria, 26 in Malmö and 52 in Solna. Four children were excluded: 2 did not fulfil the diagnostic criteria and 2 had parents with difficulties understanding Swedish. The median age of the children included was 7 (range 2–15) years. Twenty-eight (16 males, 12 females) were between 2 and 4 years, 18 (8 males, 10 females) between 5 and 8 years, and 32 (14 males, 18 females) between 9 and 15 years. Fifty-three of 78 (68%) were new referrals, while 25 of 78 (32%) were follow-up visits. Three of the children were currently participating in the department’s eczema school. Two had just started and the third was in the middle.
Eczema
For 70 of 78 children (90%), ongoing treatment for eczema was reported (e.g. emollients, topical glucocorticoids, topical calcineurin inhibitors, oral antihistamines, or phototherapy). The remaining children, all new referrals, used no treatment.
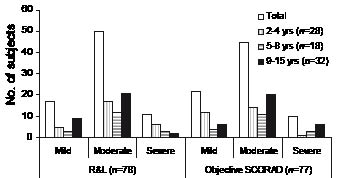
The eczema severity scored according to Rajka & Langeland and objective SCORAD is shown in Fig. 1. The mean value ± standard deviation (SD) for the Rajka & Langeland score was 6.1 ±1.6 in children 2–4 years of age, 5.7 ±1.5 in children 5–8 years, and 5.7 ±1.5 in children 9–15 years. The mean value ± SD for objective SCORAD was 21.3 ±11.7 in children 2–4 years of age, 26.7 ±14.8 in children 5–8 years, and 26.4 ±13.0 in children 9–15 years. The mean value ±SD for total SCORAD was 29.5 ±15.6 in children 2–4 years of age, 34.6 ±17.7 in children 5–8 years, and 32.7 ±14.2 in children 9–15 years. The mean IDQOL dermatitis severity score, which reflects the points of view of parents whose children are 2–4 years old, was 2.1 ±1.2.
Fig. 1. Eczema severity scores according to Rajka & Langeland (R&L) and objective SCORAD in the participating children. The number of persons is given. For eczema scoring see paragraph on Examination under Methods.
The correlation coefficient between the Rajka & Langeland score and the objective SCORAD was 0.35 (p <0.002). The correlation coefficients between the mean dermatitis severity score as reported by the parents in the IDQOL and the Rajka & Langeland or the objective SCORAD score were 0.20 (not significant) and 0.64 (p <0.001), respectively.
Other disorders
A history of concomitant disorders for the previous year was reported in 42 of 78 children (54%). These disorders were asthma in 15 (19%), allergic rhinoconjunctivitis in 16 (21%), food allergy/intolerance in 28 (36 %) and urticaria in 13 (17%). No child reportedly had any endocrine/metabolic, cardiac, neurological or musculoskeletal disorder.
Quality of life
The total scores of the different QoL questionnaires are shown in Table I. There was no significant gender difference.
Table I. Total quality of life scores as measured with the different questionnaires, and the correlations (Spearman’s rank order) between IDQOL/CDLQI and DFI, and between quality of life scores and eczema severity scores
| | IDQOL (2–4 years of age) | CDLQI (5–15 years of age) | DFI (Parental view) |
| Number | 28 | 50 | 77a |
| Median (range) | 7.0 (1–18) | 6.0 (2–18) | 8.0 (0–27) |
| Mean (±SD) | 8.6 (±5.3) | 7.1 (±4.4) | 8.3 (±6.0) |
| Correlation with DFI | 0.78 (p <0.01) | 0.44 (p <0.01) | – |
| Correlation with Rajka & Langeland eczema severity score | 0.48 (p <0.05) | 0.31 (p <0.05) | 0.44 (p <0.01) |
| Correlation with objective SCORAD eczema severity score | 0.30 (p >0.05) | 0.18 (p >0.05) | 0.18 (p >0.05) |
aData from one subject missing due to incomplete questionnaire.
IDQOL: Infants’ Dermatitis Quality of Life Index; CDLQI: Children’s Dermatology Life Quality Index; DFI: Dermatitis Family Impact Questionnaire.
The median total IDQOL score was 7.0 (range 1–18). There was no significant difference between infants with eczema and infants with eczema and asthma, allergic rhinoconjunctivitis, food allergy/intolerance or urticaria (AAFU) (mean±SD: 9.80 ± 5.34 vs. 7.31 ± 5.07, p=0.2). The three items in the IDQOL questionnaire that showed the highest scores (mean ± SD), i.e. the most negative impact on QoL, were “itching and scratching” (1.75 ± 0.97), “time to get the child to sleep” (1.11 ± 0.74), and “the child’s mood” (1.00 ± 0.86). The lowest-scored item was “uncomfortable dressing and undressing” (0.50 ± 0.88).
The median total CDLQI score was 6.0 (range 2–18). Again, there was no significant difference between children with eczema and children with eczema and AAFU (mean ± SD: 6.96 ± 4.17 vs. 7.26 ± 4.71, p=0.97). The mean score was higher for the younger children than the older ones (8.38 ± 3.99 for 5–8 years of age vs. 6.38 ± 4.49 for 9–15 years; p <0.05). The three items in the CDLQI questionnaire that showed the highest score (mean ± SD), were “itchy, scratchy, sore or painful” skin (1.66 ± 0.72), “problem with skin treatment” (1.18 ± 0.94), and “embarrassed, self-conscious, upset or sad because of the skin” (0.86 ± 0.93). The item that showed the lowest score was “change or worn different or special clothes/shoes because of the skin” (0.50 ± 0.88).
The median of the total DFI score was 8.0 (range 0–27). Here the children with eczema and AAFU had a higher total score than those with eczema without AAFU (mean ± SD: 9.59 ± 6.15 vs. 6.78 ± 5.53; p <0.03). The mean score was highest for the youngest age group (10.30 ± 6.74 for 2–4 years of age, 9.28 ± 5.89 for 5–8 years, 6.00 ± 4.65 for 9–15 years; p <0.05). The three items in the DFI questionnaire affecting QoL (mean ± SD) most negatively were “main carer’s helping with the child’s treatment” (1.13 ± 0.89), “child’s eczema causing tiredness or exhaustion in parents/carers” (1.08 ± 1.00), and “costs and expenditure related to treatment, clothes, etc.” (1.05 ± 0.92). The item with the least negative effect on the family was “effect of time spent on shopping because of the child’s eczema” (0.50 ± 0.88).
As shown in Table I, there was a significant correlation between the DFI and the IDQOL or CDLQI scores. Furthermore, the Rajka & Langeland eczema severity score correlated significantly with all total QoL scores, which was not the case with the objective SCORAD.
DISCUSSION
This Swedish study of the impact of childhood eczema on children’s’ QoL and on their families was carried out in a hospital setting. Special attention was paid to the recruitment of children of various ages and to careful mapping of the eczema severity and concomitant disorders. In addition, care was taken to obtain the children’s and the parents’ scores separately. Hence, the parents left the room when the children (>5 years of age) did their scoring, and vice-versa.
The study demonstrates and confirms that eczema impairs the children’s QoL and also affects their families. We were unable to detect any significant gender difference in QoL scores. On the other hand, the child’s age did influence the scores: the younger the child, the higher the DFI and CDLQI, which implies good agreement between the children’s own QoL rating and the parents’ assessment of the impact of eczema on the family. As may be expected, the DFI score was significantly higher for children with eczema and AAFU than for those with eczema, but without AAFU. Interestingly, no such difference occurred for the children’s own rating.
New referrals and patients on follow-up visits were invited to participate. The mean total IDQOL score was 8.6, agreeing with a study by Lewis-Jones et al. (9) (mean 7.9), but higher than in that of Chinn et al. (18) (mean 5.9). However, in the former the patients were recruited from a paediatric dermatology clinic, while in the latter they were recruited from two general practices. It seems reasonable that patients referred to a specialist unit would score higher than patients from general practice. The three items showing the highest scores in our study referred to “itching and scratching”, “time to get the child to sleep” and “the child’s mood”. In fact, these were the same as in the study from the UK (9), indicating a similar impact pattern of eczema on QoL in Swedish and English children and families. The mean total CDLQI score was 7.1 in our study, slightly lower than in comparable studies with their means of 7.7 (8) and 7.9 (19). Our mean total DFI score was 8.3, with the highest score for the youngest group (2–4 years). In a similar study including children aged between 6 months and 12 years attending a hospital clinic in the UK, the DFI score was 9.6 (10), whereas in a community-based study of the same age group it was only 2.4 (19). Although eczema is the most common chronic disease in children, there have been very few QoL assessments, especially for the youngest children. Most investigations estimate QoL in connection with the introduction of different treatment modes (11). In eczema care there is a need to learn and understand more about the impact of skin disease in every aspect of life, for both the child and his or her parents.
This study showed clear correlations between IDQOL, CDLQI, DFI and the eczema severity score estimated with the Rajka & Langeland scoring; but not with the objective SCORAD. This may be because the former covers a longer period and has fewer scale steps, increasing the probability of a significant correlation in a study population of the size of ours. The relationship between QoL assessments and measurements of disease severity needs much more attention. To the best of our knowledge there is only one publication in adults on the correlation between QoL assessment and eczema severity estimated with the easy-to-use Rajka & Langeland scoring (20). A correlation between DFI and the severity of eczema rated with a modified SCORAD has been observed previously in out-patients investigated twice with an interval of 6 months (19). In another study, DLQI and CDLQI correlated with eczema severity assessed with objective SCORAD (21).
Our study was the first to use the DFI in the Swedish language. We found the Swedish DFI and the IDQOL and CDLQI easy to use in the clinical situation, and the children and their parents had no difficulties understanding the questions. When comparing the situation for patients in different countries, it is of great importance to use the same QoL instruments (22), correctly adapted to the language spoken in the population studied. The same holds true when planning the allocation of healthcare resources to the care of eczema and other skin diseases. When comparing QoL impact between different skin diseases and other diseases (e.g. diabetes, asthma), general QoL questionnaires are needed (23).
In conclusion, our study showed that childhood eczema impaired the QoL of Swedish children and their families. This is in line with other Northern European studies. There was no significant gender difference in scores, but the younger the child, the more the QoL of the family was impaired. Eczema with concomitant asthma, food allergy/intolerance, allergic rhinoconjunctivitis or urticaria also impaired QoL more than did eczema alone.
ACKNOWLEDGEMENTS
We thank all the children and parents for their participation in the study. The valuable assistance of Therese Sterner, nurse at the Department of Dermatology, Malmö University Hospital MAS, and the staff at the Paediatric Dermatology Unit of the Karolinska University Hospital, Solna, is acknowledged. We also thank Karin Einarsson, medical student, and Nuray Güner, medical statistician, for help with data processing and medical statistics, respectively.
The study was supported by grants from the Swedish Asthma- and Allergy Association’s Research foundation, the Edvard Welander-Finsen Foundation, the First Mayflower Campaign for Children’s Health, Stockholm County Council, and the Sunnerdahl Disability Foundation.
REFERENCES
1. Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol 2004; 113: 832–836.
2. Åberg N, Hesselmar B, Åberg B, Eriksson B. Increase of asthma, allergic rhinitis and eczema in Swedish schoolchildren between 1979 and 1991. Clin Exp Allergy 1995; 25: 815–819.
3. Broberg A, Svensson A, Borres MP, Berg R. Atopic dermatitis in 5–6-year-old Swedish children: cumulative incidence, point prevalence, and severity scoring. Allergy 2000; 55: 1025–1029.
4. Finlay AY. Measurement of disease activity and outcome in atopic dermatitis. Br J Dermatol 1996; 135: 509–515.
5. Wahlgren CF. Itch and atopic dermatitis: an overview. J Dermatol 2000; 26: 770–779.
6. Reid P, Lewis-Jones MS. Sleep difficulties and their management in preschoolers with atopic eczema. Clin Exp Dermatol 1995; 20: 38–41.
7. Finlay AY. Quality of life measurement in dermatology: a practical guide. Br J Dermatol 1997; 136: 305–314.
8. Warschburger P, Buchholz HT, Petermann F. Psychological adjustment in parents of young children with atopic dermatitis: which factors predict parental quality of life? Br J Dermatol 2004; 150: 304–311.
9. Lewis-Jones MS, Finlay AY. The children’s dermatology life quality index (CDLQI): initial validation and practical use. Br J Dermatol 1995; 132: 942–949.
10. Lewis-Jones MS, Finlay AY, Dykes PJ. The Infants’ Dermatitis Quality of Life Index. Br J Dermatol 2001; 144: 104–110.
11. Lawson V, Lewis-Jones MS, Finlay AY, Reid P, Owens RG. The family impact of childhood atopic dermatitis: the Dermatitis Family Impact Questionnaire. Br J Dermatol 1998; 138: 107–113.
12. http://www.dermatologyorg.uk/portal/quality.
13. Williams HC, Burney PGJ, Pembroke AC, Hay RJ. Validation of the U.K. diagnostic criteria for atopic dermatitis in a population setting. Br J Dermatol 1996; 135: 12–17.
14. Williams HC. Diagnostic criteria for atopic dermatitis. Lancet 1996; 348: 1391–1392.
15. Rajka G, Langeland T. Grading of the severity of atopic dermatitis. Acta Derm Venereol 1989; 144: 13–14.
16. European Task Force on Atopic Dermatitis. Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology 1993; 186: 23–31.
17. Kunz B, Oranje OP, Labréze L, Stalder J-F, Ring J, Taieb A. Clinical validation and guidelines for the SCORAD index: consensus report of the European task force on atopic dermatitis. Dermatology 1997; 195: 10–19.
18. Chinn DJ, Poyner T, Sibley G. Randomized controlled trial of a single dermatology nurse consultation in primary care on the quality of life of children with atopic eczema. Br J Dermatol 2002; 146: 432–439.
19. Ben-Gashir MA, Seed PT, Hay RJ. Are quality of family life and disease severity related in childhood atopic dermatitis? J Eur Acad Dermatol Venereol 2002; 16: 455–462.
20. Higaki Y, Kawamoto K, Kamo T, Ueda S, Arikawa J, Kawashima M. Measurement of the impact of atopic dermatitis on patients’ quality of life: a cross-sectional and longitudinal questionnaire study using the Japanese version of Skindex-16. J Dermatol 2004; 31: 977–982.
21. Holm EA, Wulf HC, Stegmann H, Jemec GB. Life quality assessment among patients with atopic eczema. Br J Dermatol 2006; 154: 719–725.
22. Lewis V, Finlay AY. 10 years experience of the Dermatology Life Quality Index (DLQI). J Investig Dermatol Symp Proc 2004; 9: 169–180.
23. Beattie PE, Lewis-Jones MS. A comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. Br J Dermatol 2006; 155: 145–151.